Showing 1 to 15 of 2114 results
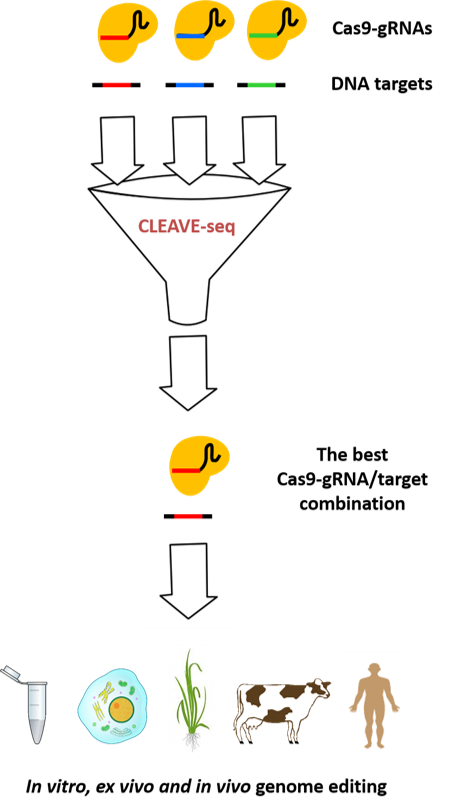

CLEAVE-seq: technology for determination of on- and off-target sites for DNA endonucleases
Patents for licensing Vilnius University

Composition Containing Arylnaphthalene Lignan Derivative For Preventing And/Or Treating Dementia
Innovative Products and Technologies CSIR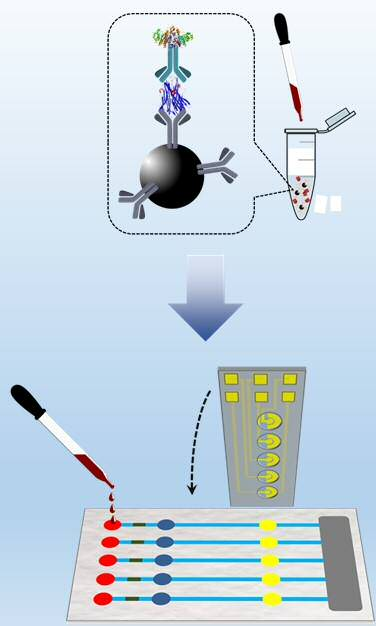

Point-of-care analytical device for rapid and multiplexed detection of biomarkers
Patents for licensing Consejo Superior de Investigaciones Científicas

Sustainable gypsum prefabricated from polyurethane foam residue
Patents for licensing UNIVERSIDAD DE BURGOS

Antibody for Preventing/Treating Secondary Respiratory Infections
Patents for licensing Yeda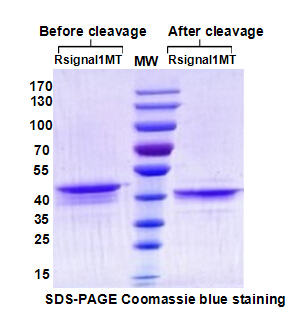

Insect Baculovirus Expression Vector System
Research Services and Capabilities Leading Biology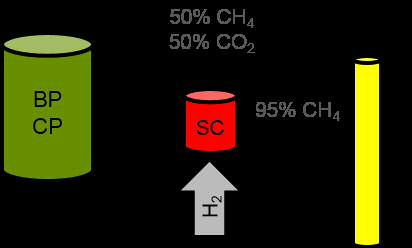

Catalyst for the upgrading of raw biogas
Patents for licensing Consejo Superior de Investigaciones Científicas

Model combination method (ensembles). Construction of "Rotation Forest" classifform-control form-control iers and regressors
Research Services and Capabilities UNIVERSIDAD DE BURGOS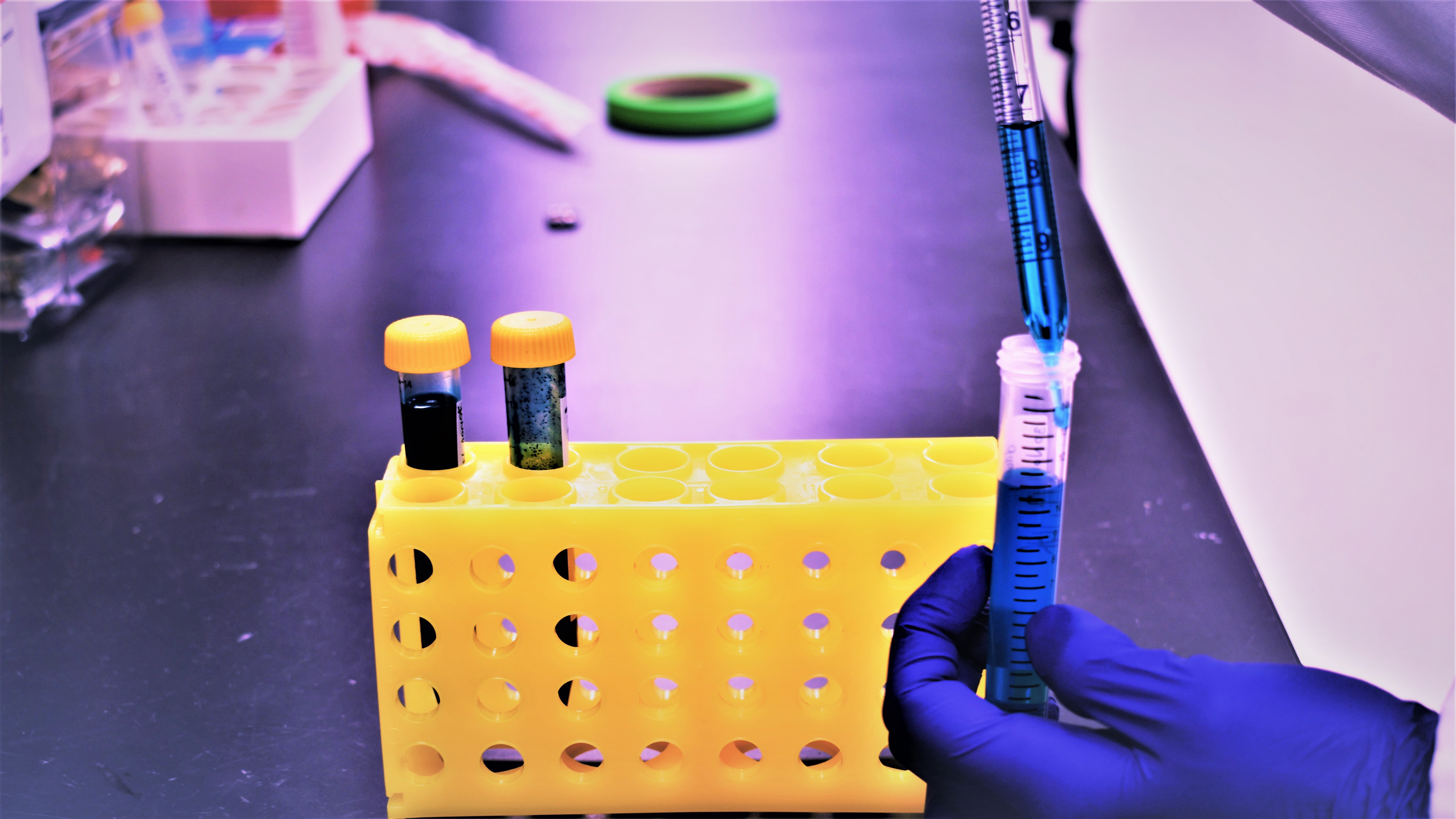

Food safety, quality, authenticity and consulting services
Research Services and Capabilities Avazyme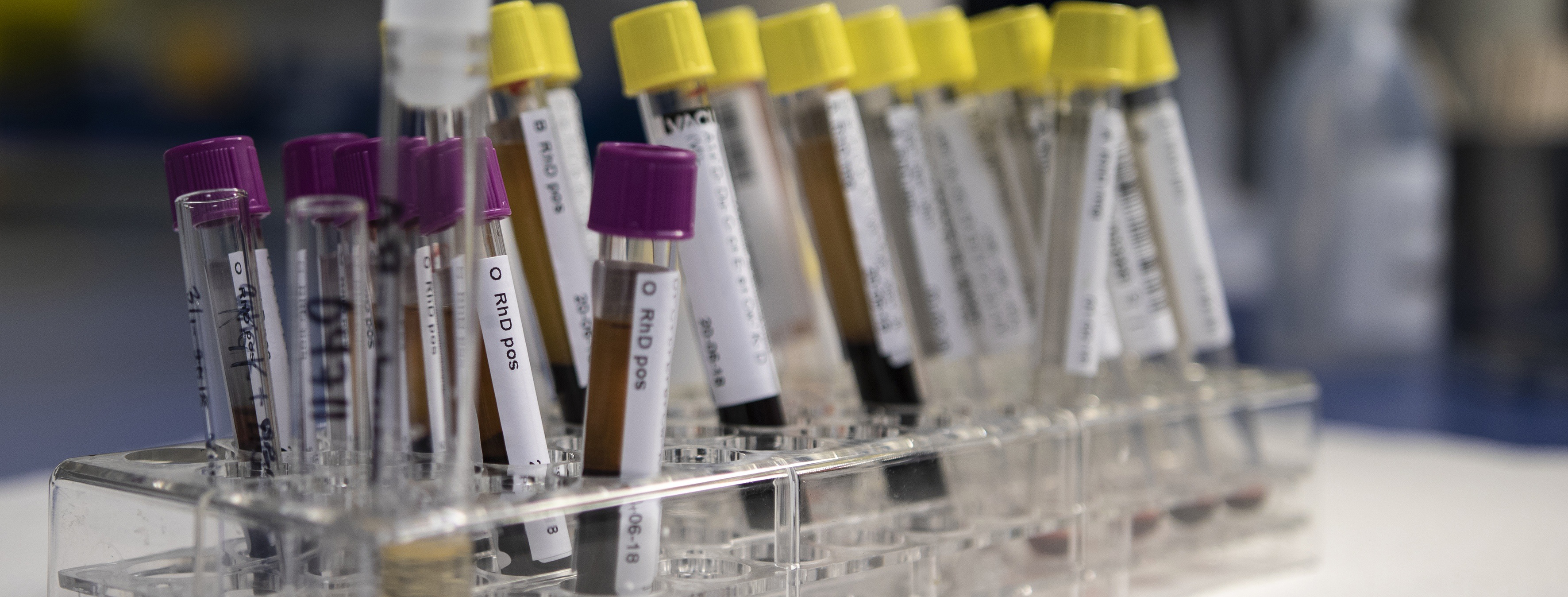

Magnetic nanoparticles for selective enrichment of proteins in complex mixtures
Patents for licensing UATEC - Unidade de Transferência de Tecnologia

US9369082 (B2) - Solar power mobile charging station
Patents for licensing Syncoda Technologies

Novel transparent polymer nanocomposites based on PMMA and inorganic semiconductor nanoparticles for optoelectronics applications
Knowhow and Research output Cracow University of Technology![Establishment, repair and delating procedure of disjoint multiple paths, redirection of frames and network bridge. Multiple disjoi[…]](https://static9.innoget.com/uploads//83a144de0398f6b9af880b08c84b86b5b1cd0eca.jpg)

Establishment, repair and delating procedure of disjoint multiple paths, redirection of frames and network bridge. Multiple disjoi[…]
Patents for licensing Universidad de Alcalá-OTRI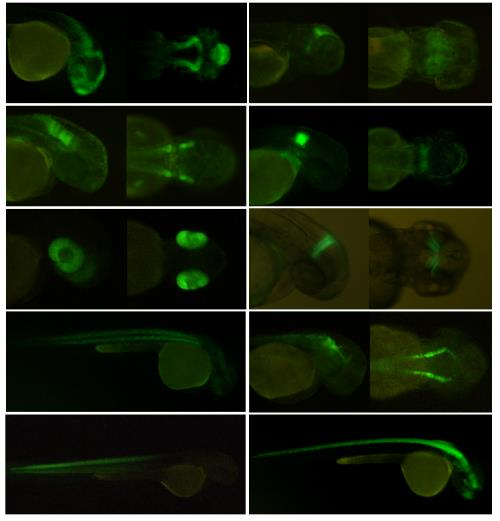

Kit for the systematic generation of transgenic lines for transcriptomic analyses
Patents for licensing Consejo Superior de Investigaciones Científicas

