Showing 1 to 15 of 2456 results

Polymer Conductor with properties according to your needs
Innovative Products and Technologies UNIVERSIDAD DE BURGOS

Gas Turbine
Innovative Products and Technologies CSIR

Coverage of carbon nanotubes for use as an anchoring system for nano and micrometer devices with therapeutic activity
Patents for licensing CINBIO

CREACTIVES - AI for Supply Chain Management
Innovative Products and Technologies EIT Digital

Nanoencapsulated ginger extract for food applications
Innovative Products and Technologies UATEC - Unidade de Transferência de Tecnologia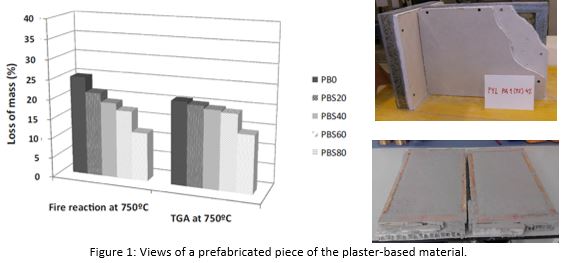

New fire resistant plaster based material
Innovative Products and Technologies UNIVERSIDAD DE BURGOS

High UV efficiency lighting
Innovative Products and Technologies University of Waterloo

Ezrin Inhibitors in Cancer Treatment
Innovative Products and Technologies Georgetown University

Transformation of biomass waste to obtain catalysts of interest to the chemical industry
Patents for licensing Universidad de Alicante

Industrial production of humic and fulvic acid from biomethan digestate
Innovative Products and Technologies Luxembourg Institute of Science and Technology (LIST)

Precision medicine for obesity treatment
Patents for licensing IBIMA-Plataforma Bionand

EasyPlan project management system
Innovative Products and Technologies University of Waterloo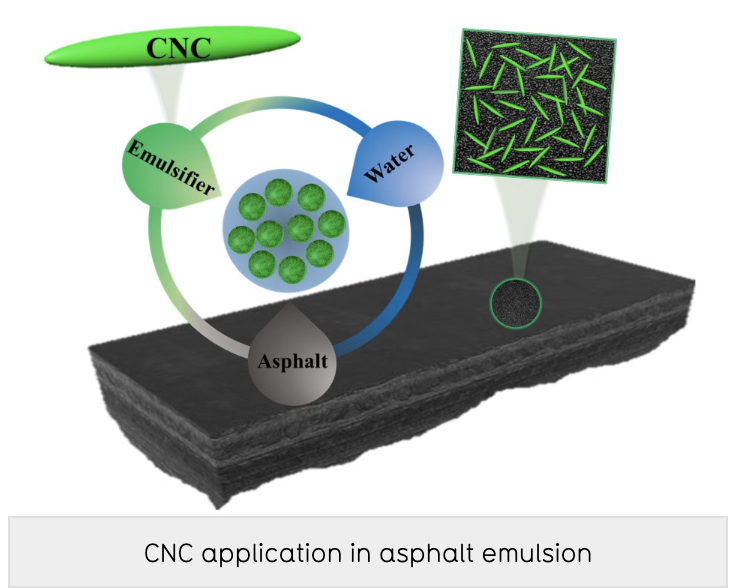

A stable, environmentally friendly emulsifier for asphalt binder
Innovative Products and Technologies University of Waterloo

Innovative device for electro-mechanical stimulation for tissue engineering applications
Patents for licensing Germans Trias i Pujol Health Sciences Research Institute