Showing 1 to 15 of 2456 results


Innovative heat-reflective coating for indoor application
Innovative Products and Technologies Laser Consult Ltd.

Methods for treatment, diagnosis, and early detection of dry age-related macular degeneration
Innovative Products and Technologies Georgetown University

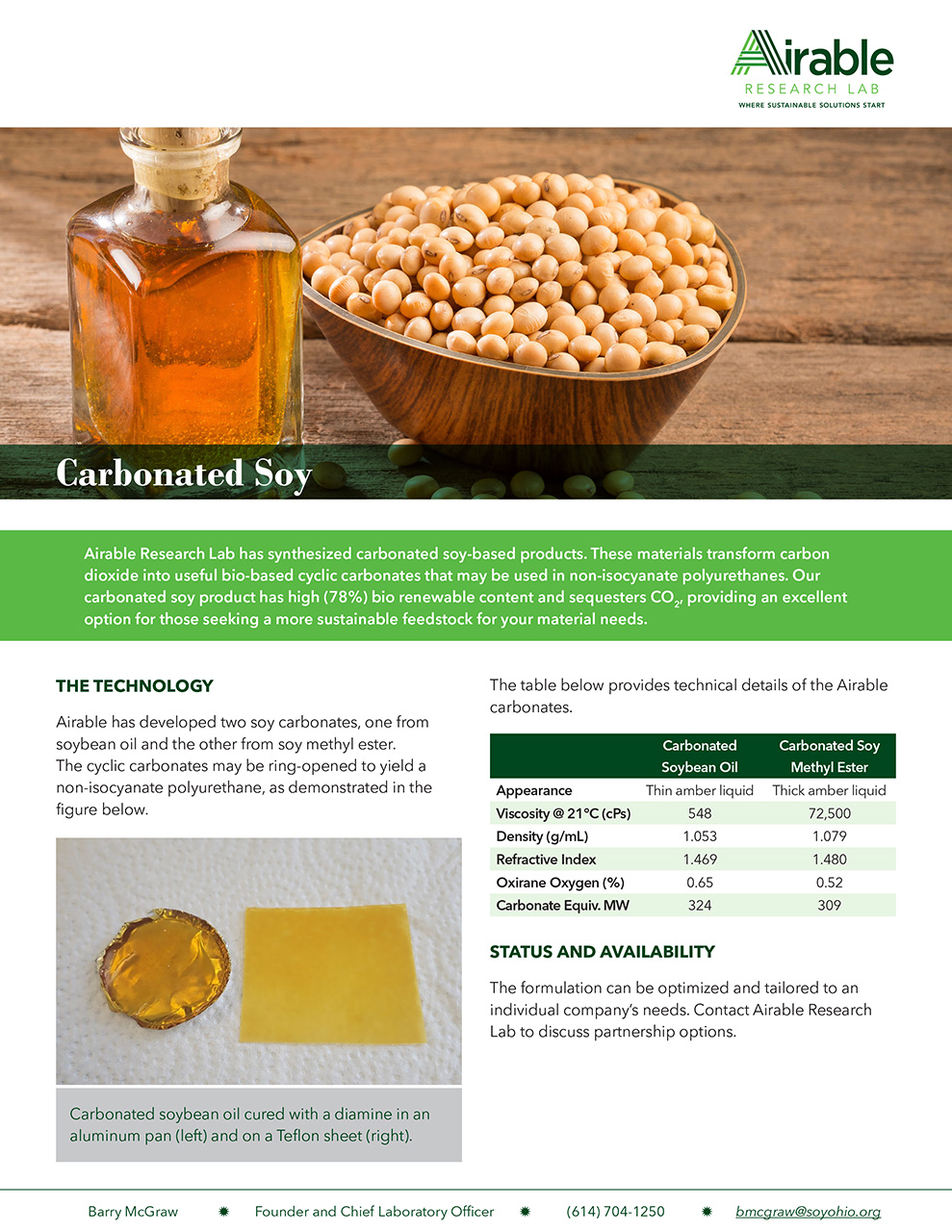
Vegan Thrive, A Capsule-in-Capsule Supplement for Vegans
Investment Opportunities in Startups and Spinoffs Robert DiSilvestro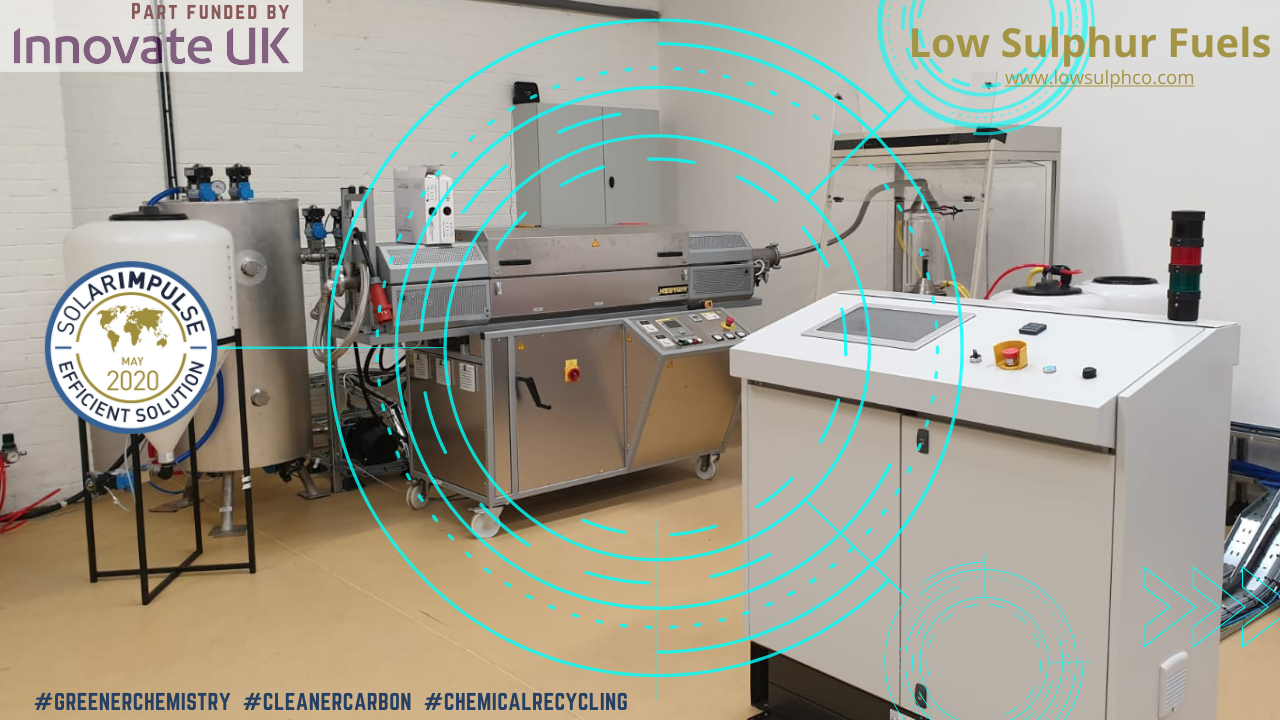
LSF: B2B Chemtech, UK & NL, Angel round with lead investor, ≈40% funding secured
Investment Opportunities in Startups and Spinoffs Low Sulphur Fuels Ltd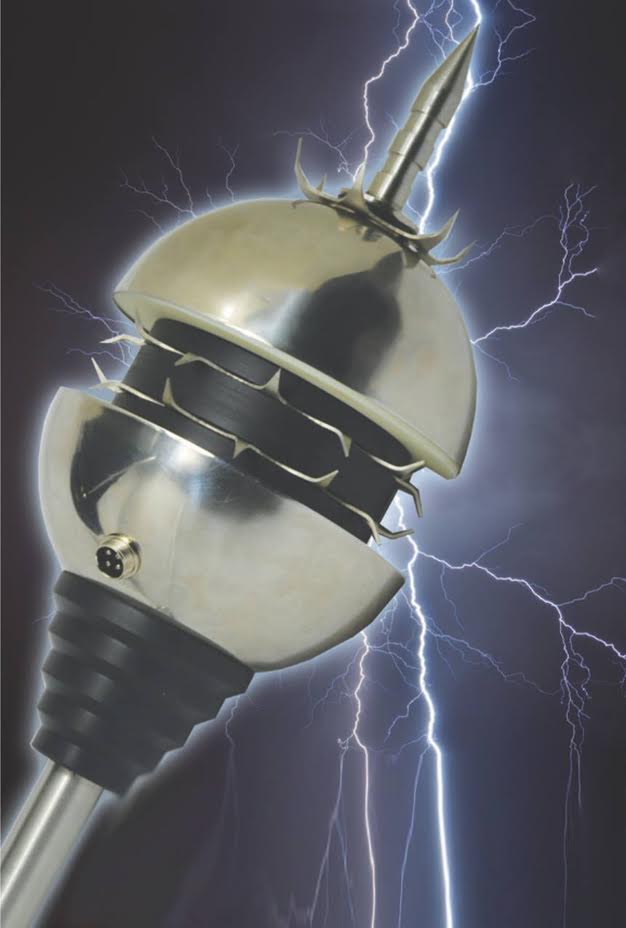

Lightning conductor with upgradeable modular structure
Innovative Products and Technologies Laser Consult Ltd.

Nitric Oxide Chewing Gum Delivering in the Oral Cavity
Innovative Products and Technologies Per Os Biosciences

Wide Area Surveillance Platform (WASP)
Innovative Products and Technologies CSIR

LORIOT - Secure and scalable long-range infrastructure provider for IoT
Innovative Products and Technologies EIT Digital

High UV efficiency lighting
Innovative Products and Technologies University of Waterloo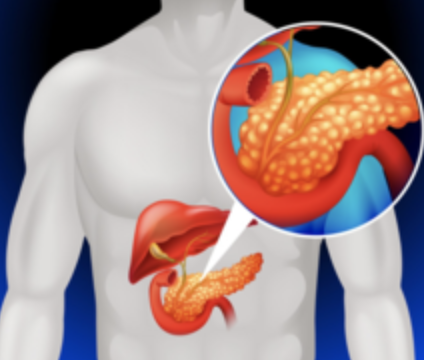

Targeted LNA Gapmer Therapy for Pancreatic Cancer
Innovative Products and Technologies Georgetown University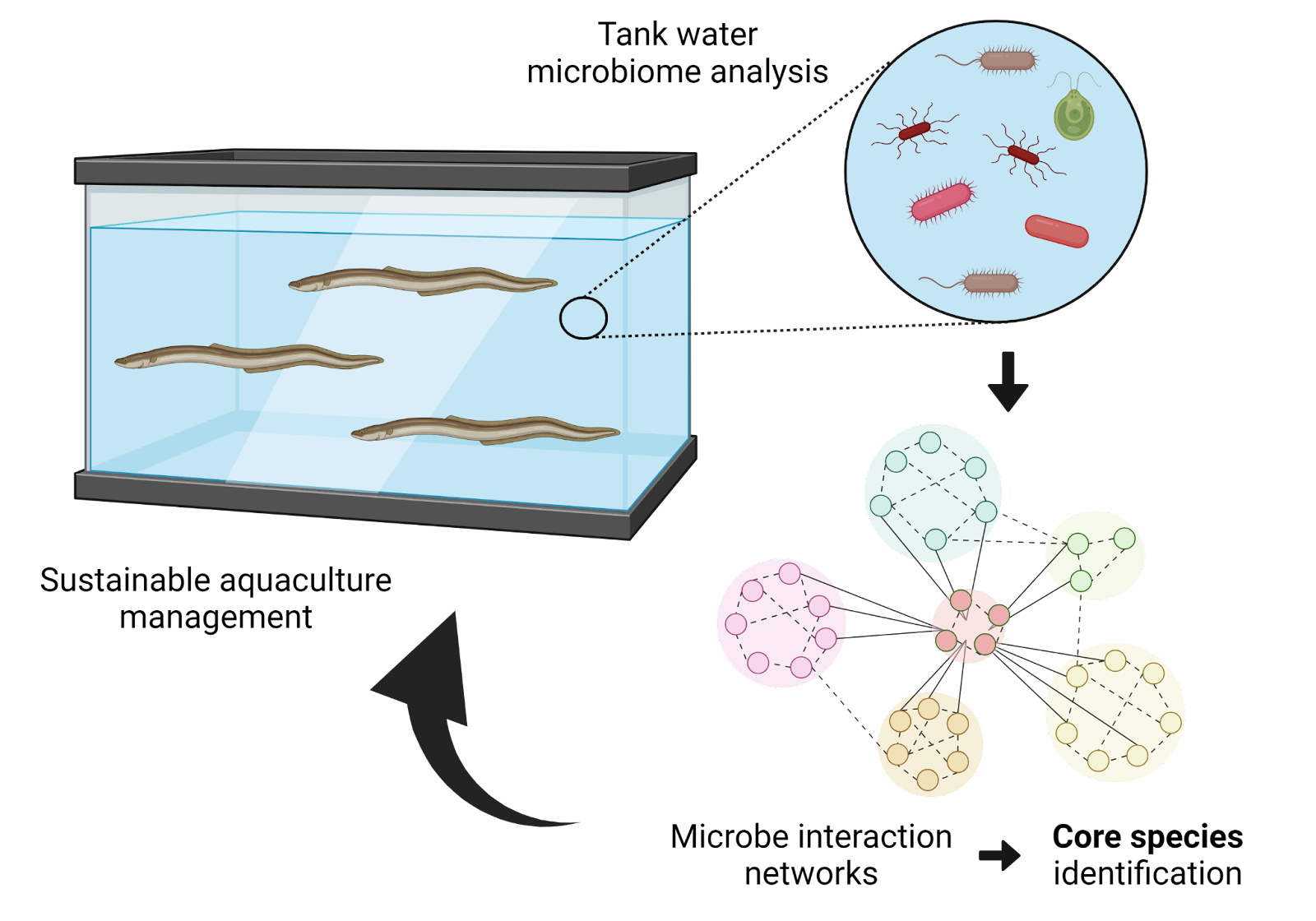

Microbial networks for sustainable aquaculture
Innovative Products and Technologies Kyoto University

Diagnosis and Prognosis of Inflammatory Bowel Diseases Using Host RNA Sequencing
Patents for licensing Yeda

Industrial production of humic and fulvic acid from biomethan digestate
Innovative Products and Technologies Luxembourg Institute of Science and Technology (LIST)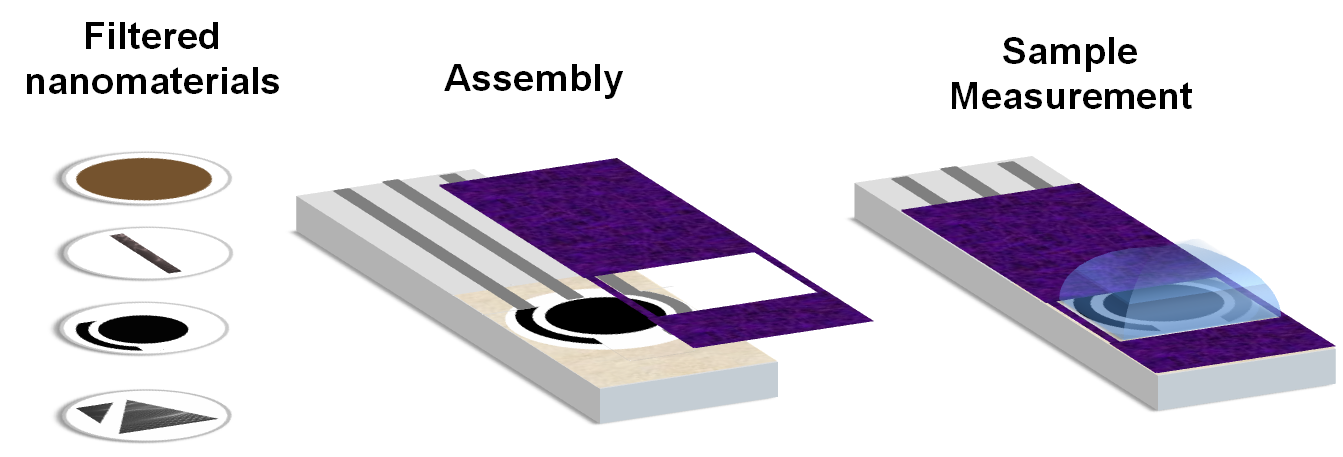

Disposable electrodes based on filtered nanomaterials.
Patents for licensing Universidad de Alcalá-OTRI