Showing 1 to 15 of 2472 results


Automated multi sensory rooms
Innovative Products and Technologies UNIVERSIDAD DE BURGOS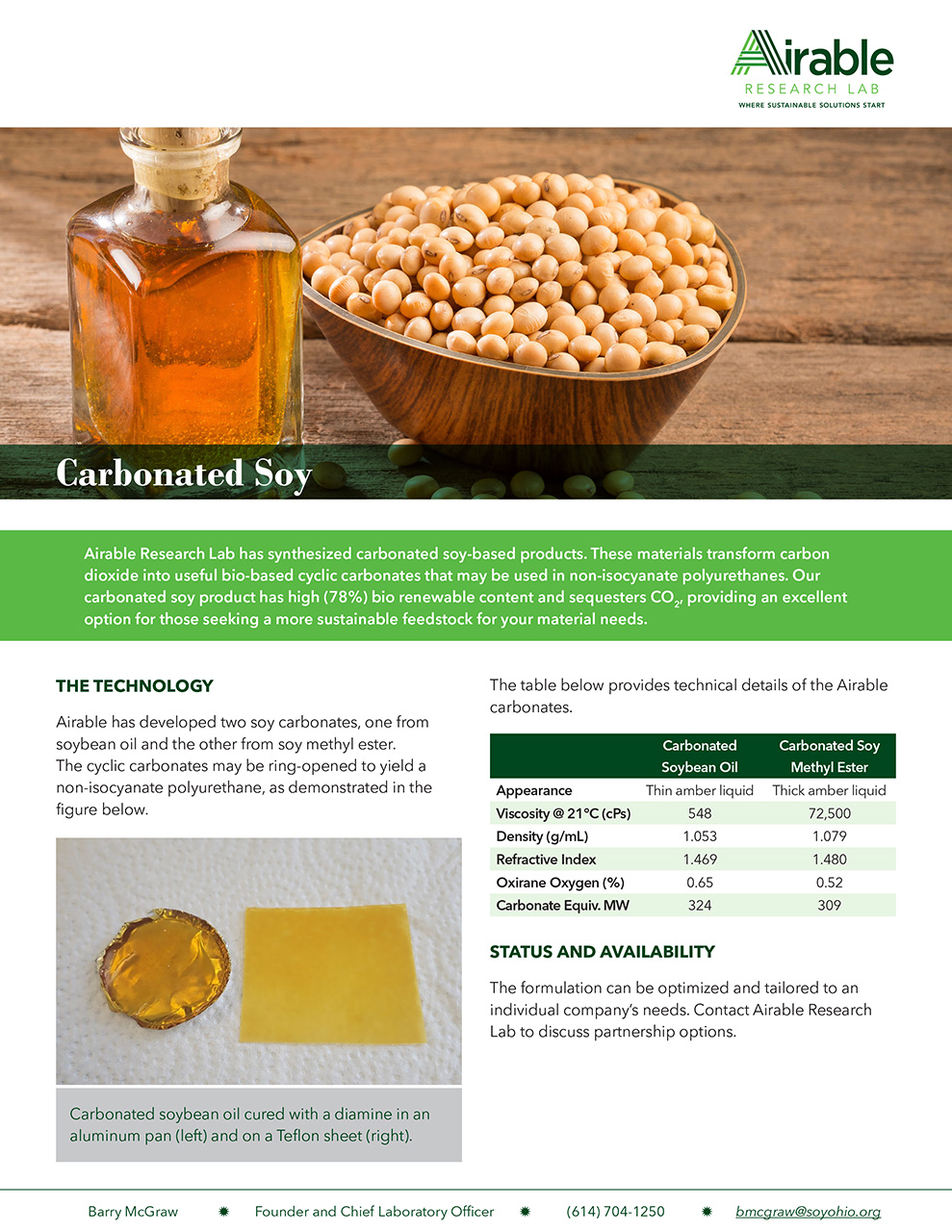

Carbonated Soy-Based Products That Transform Carbon Dioxide
Innovative Products and Technologies Airable Research Lab, business line of Ohio Soybean Council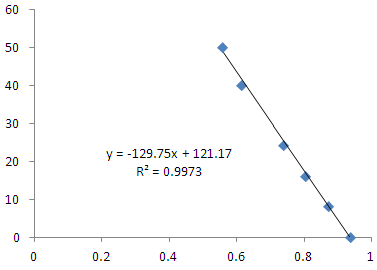

Protein Expression in E coli
Research Services and Capabilities Leading Biology

Detector based on a film for in situ Iron, Aluminium and Mercury measures
Patents for licensing UNIVERSIDAD DE BURGOS

Know-how in Sodium ion/sodium metal batteries
Patents for licensing Universidad de Alicante

Lighting for safer landing – a portable landing light system
Innovative Products and Technologies CSIR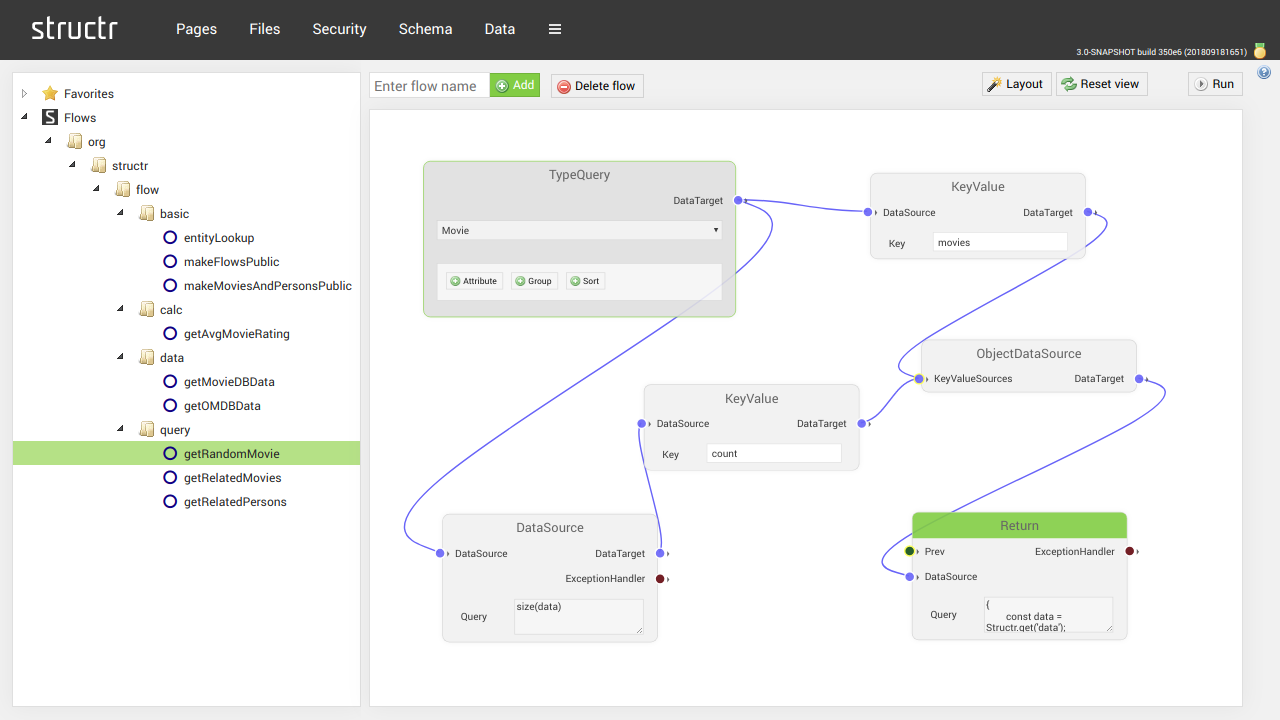

STRUCTR - Low-Code Dev. Platform combined with Graph Technology
Innovative Products and Technologies EIT Digital

Advanced electro catalyst for the batteries
Innovative Products and Technologies Nu innovations Inc

Technology and innovation in food and beverage
Innovative Products and Technologies SENAI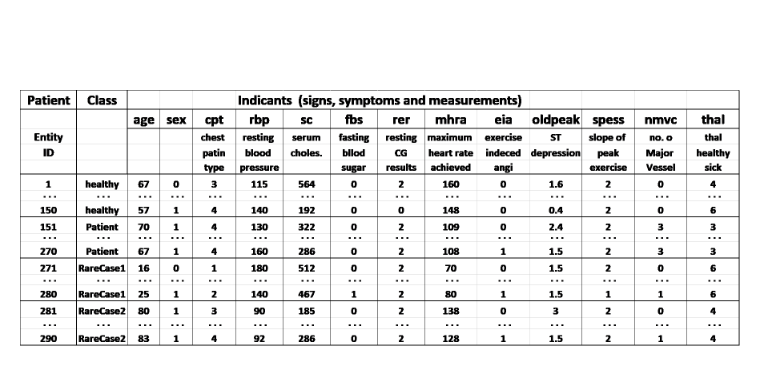

Pattern Discovery and Disentanglement (PDD) AI software for deep knowledge discovery from relational data
Innovative Products and Technologies University of Waterloo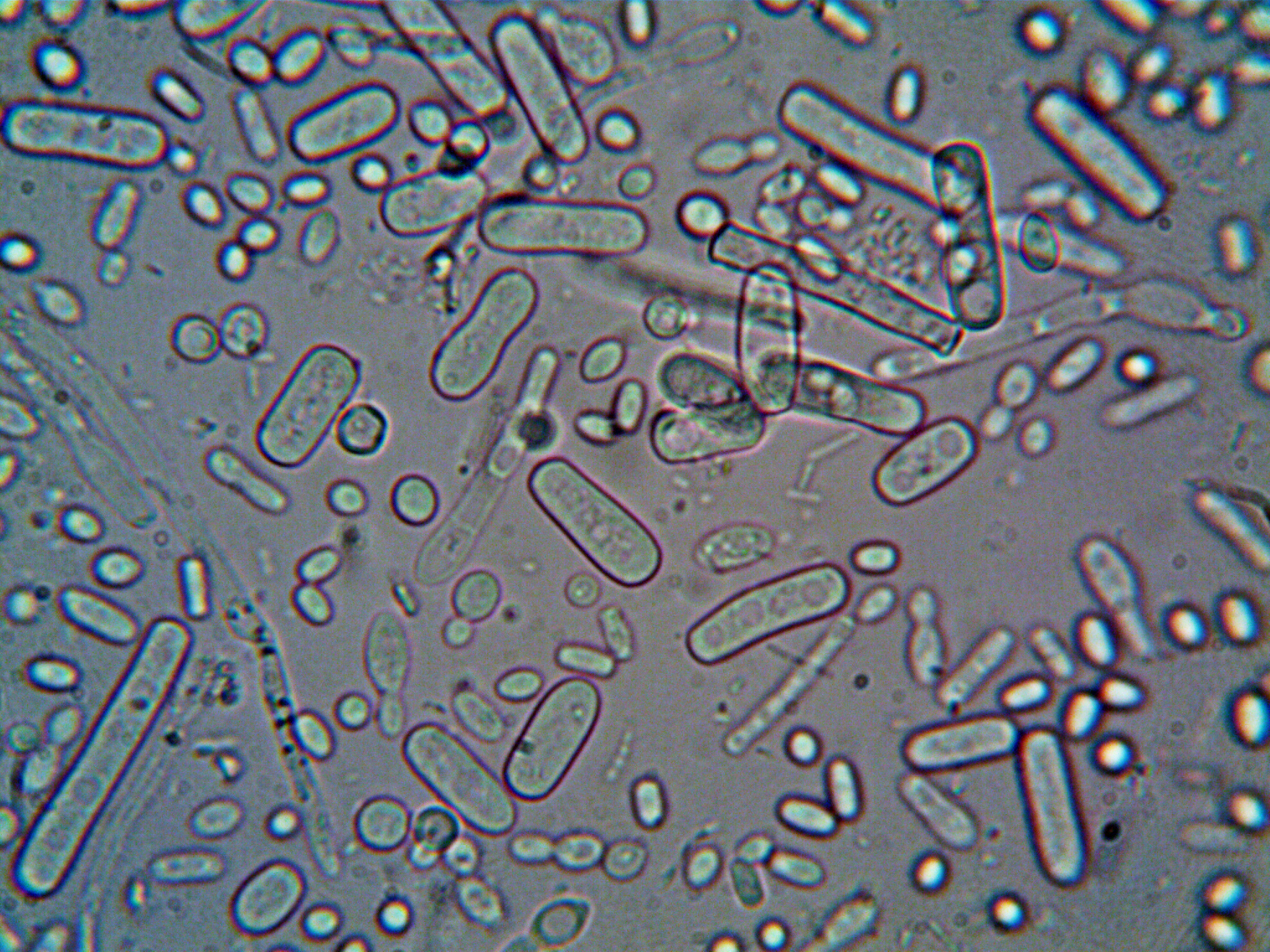

The use of microorganisms in the plant’s production
Patents for licensing Jagiellonian University

Prognostic biomarker in COVID-19 patients.
Patents for licensing IBIMA-Plataforma Bionand

A novel mild and efficient method to prepare ε-Caprolactam precursor of Nylon 6
Patents for licensing University of Vigo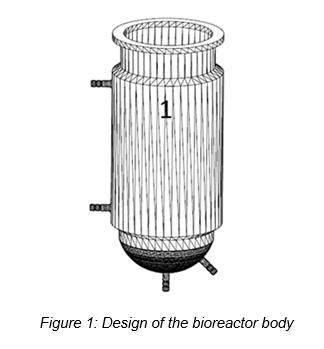

New bioreactor for growing plant cell culture in suspension
Patents for licensing UNIVERSIDAD DE ALICANTE

