Showing 1 to 15 of 2455 results


Nitrate ions removal from water
Patents for licensing UNIVERSIDAD DE BURGOS

Automated multi sensory rooms
Innovative Products and Technologies UNIVERSIDAD DE BURGOS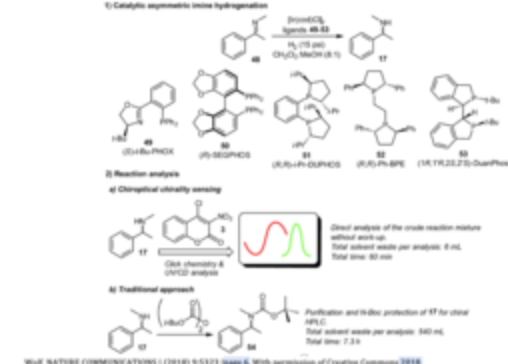

Chirality Sensing with Molecular Click Chemistry Probes
Innovative Products and Technologies Georgetown University

WPGear - A mechanical gear unit
Innovative Products and Technologies Laser Consult Ltd.

Immunosensor for Detection of Inflammation in Amniotic Fluid
Innovative Products and Technologies University Hospital Hradec Králové

S-Adenosyl-L-Homocysteine Hydrolase, Recombinant for Enhanced Bioprocessing
Research Services and Capabilities Creative Enzymes

Autonomous vehicles for transport of materials in warehouse
Patents for licensing Universidad de Alicante

EnzoMeal™ - A Sustainable Food Source for Farm-Raised Fish
Innovative Products and Technologies Airable Research Lab, business line of Ohio Soybean Council

Self-compacting concrete with recycled concrete aggregate and low shrinkage
Innovative Products and Technologies UNIVERSIDAD DE BURGOS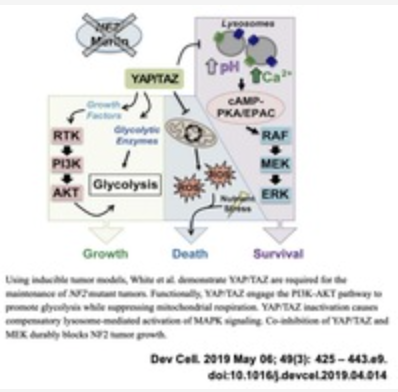

YAP/TAZ inhibitors for cancer.
Innovative Products and Technologies Georgetown University

Unconjugated PLGA Nanoparticles in the Diagnosis and Treatment of Alzheimers Disease
Innovative Products and Technologies University of Alberta, Technology Transfer Services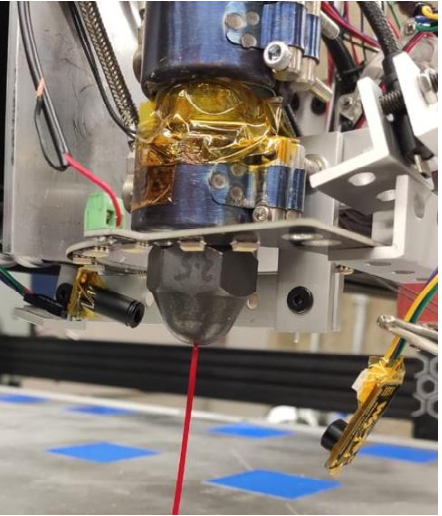

Hybrid Additive Deposition Modeling System for Recycled Plastic Products Manufacturing
Innovative Products and Technologies University of Alberta, Technology Transfer Services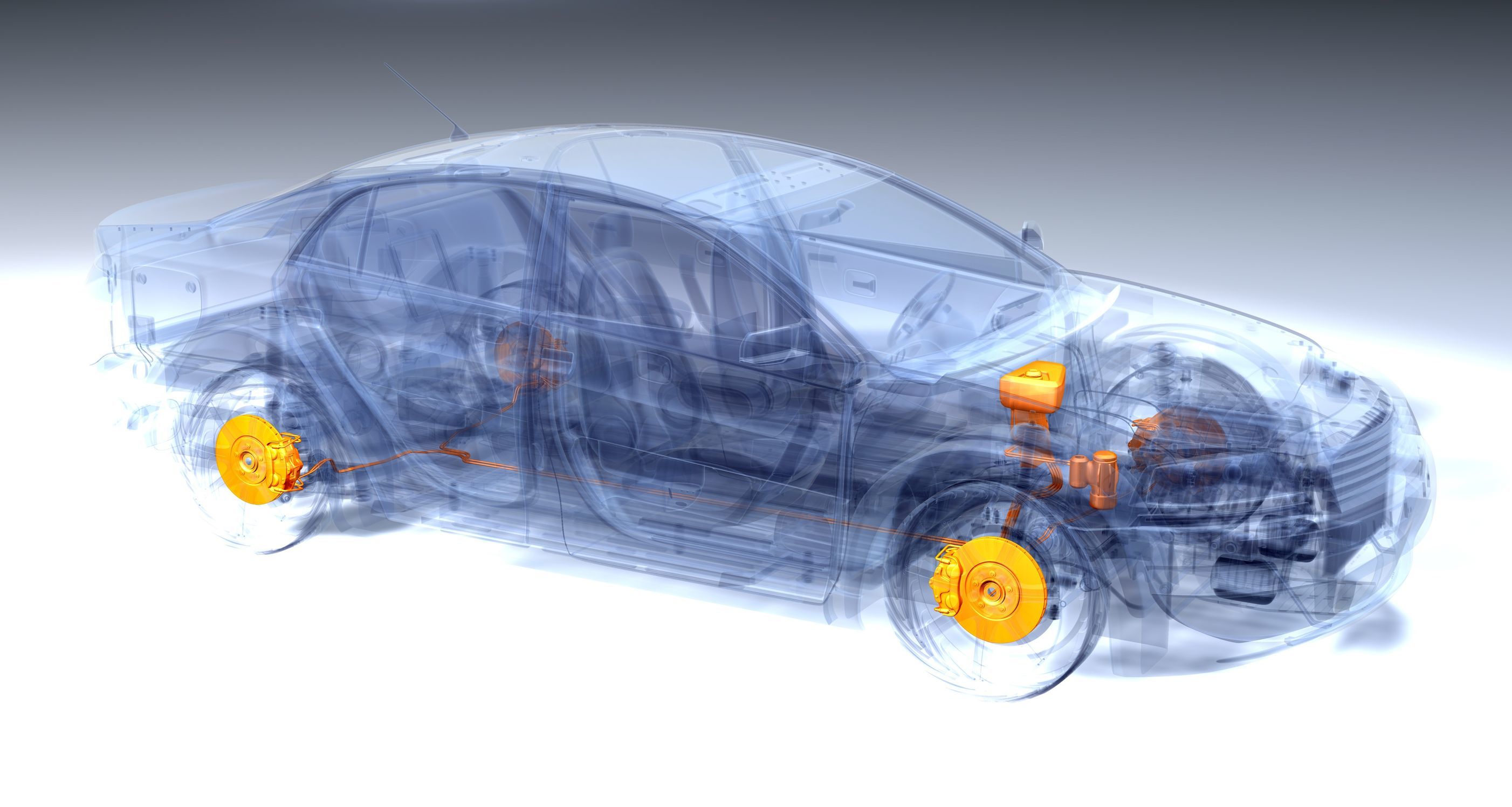

Wheel slip reset controller for brake systems
Patents for licensing University of Vigo

Strain textile sensor
Patents for licensing Universitat Politècnica de Catalunya - UPC

