Pasireotide-LAR and breast cancer chemoprevention
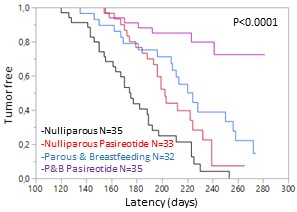
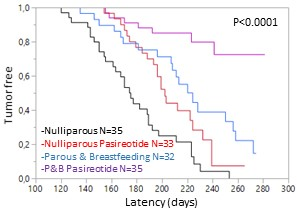
Pasireotide-LAR potentiates the protective effect of pregnancy in the Brca1/P53 deficient mice. P&B: Parous and breastbeeding mice.
Breast cancer is the most common tumor in women and a significant cause of morbidity and mortality. Its incidence increases due to epidemiological changes in society, fundamentally aging of the population, and the first pregnancy is getting later and later, often after 35 years. Prevention strategies are needed. The drugs used in current chemoprevention, such as estrogen receptor modulators, have very annoying and potentially severe side effects, such as venous thrombosis or endometrial cancer. Breast amputation surgery continues to be offered as an effective prophylactic alternative. Chemoprevention strategies with fewer side effects and less aggression are needed.
We demonstrated treatment with Pasireotide-LAR once a month in two models of genetically modified mice that develop breast cancer increases latency and decreaes the disease incidence. Furthermore, Pasireotide LAR increases the protective effect of pregnancy in Brca1 /P53-deficient mice.
These effects were associated with a decrease in epithelial proliferation and a decrease in the ductal epithelial area. This decrease in the ductal component was associated with low tumor susceptibility in mice.
Main innovations and advantages
· Pasireotide LAR is more comfortable to administer than current chemoprevention drugs (daily intake
· Pasireotide LAR would be administered once a month.
· Pasireotide LAR would have fewer side effects than current drugs; this would favor adherence to chemoprevention, in contrast to annoying side effects of current therapies, such us
· Tamoxifen.
· Pasireotide LAR could be given after breastfeeding (i) to enhance the protective effect of pregnancy against breast cancer; and (ii) to reduce the risk of post-pregnancy breast cancer in women over 30 and, above all, 35 years of age.