Never miss an update from Javier Montiel Bonmatí
Create your free account to connect with Javier Montiel Bonmatí and thousands of other innovative organizations and professionals worldwide
The Carbon Materials and Environment Research Group of the University of Alicante has developed a new method for obtaining carbon materials with excellent properties such as electrocatalysts in fuel cells or metal-air batteries. The process is based on the thermal treatment of polyaniline (or its derivatives) at high temperature and allows to obtain metal-free carbon materials with a high performance, in a very simple, fast way and in a single stage. These novel materials are characterized by their excellent catalytic activity and selectivity in the oxygen reduction reaction in alkaline medium, they are very stable and resistant to methanol and carbon monoxide poisoning, and above all, they stand out for their low manufacturing cost, which makes them promising candidates to replace the current commercial platinum-based catalysts. We are looking for companies interested in acquiring this technology for commercial exploitation.
Fossil fuels are commonly used to generate the energy needed to satisfy the world's energy demand. However, their excessive consumption has two important consequences: first, they are not unlimited resources, so they will be depleted in the medium term; and second, they cause serious damage to human health and to the environment (global warming, acid rain, photochemical smog, etc.).
It is essential to find new forms of energy generation that reduce the harmful effects of fossil fuels and increase the energy efficiency of the devices used.
In this sense, hydrogen-based energy systems are the great candidates for solving these problems. In fact, fuel cells have positioned themselves as the main system of the future for the automobile industry, and for the storage and use of renewable energies.
In this system, the global reaction is a consequence of the individual reactions that occur in the electrodes: in the anode, there is oxidation of the fuel (hydrogen, methanol, ethanol, etc.), and in the cathode, the reduction of the oxidizing agent (oxygen or air). The electrodes are separated by a selective ion exchange membrane.
In fuel cells, unlike batteries, fuel is continuously fed by an external source at the anode, where protons (which migrate through the membrane to the cathode to recombine with reduced oxygen species to form water, which is expelled from the cell) and electrons (which migrate through collectors to the cathode performing useful work that results in an electric current) are generated.
Currently, the performance and efficiency of fuel cells are not optimized. The main limiting factor is the use of platinum as a catalyst to accelerate electrode reactions. Platinum is a scarce metal (there is not enough platinum to satisfy the demand of the entire world vehicles fleet), it has a high cost, platinum nanoparticles agglomerate during use (which causes a significant reduction in its catalytic activity), and the presence of small traces of carbon monoxide or methanol can completely poison the catalyst, rendering it unusable.
Most of the platinum of the fuel cells (almost 90%) is used in the cathode due to the low rate of the oxygen reduction reaction, making this reaction the main limiting factor for these devices.
A major research effort is being made to obtain new materials for replacing or removing platinum from the cathode. For this, it is being studied:
1) Other alternative metals of higher abundance and lower cost: for instance, gold, silver, palladium, cobalt, nickel, iron, magnesium, chromium and vanadium, although the resulting catalyst still has a high cost, and the nanoparticles of the metals agglomerate, resulting in a loss of catalytic activity, and therefore, low durability.
2) Metal-free catalysts: this innovative type of metal-free catalysts radically reduces the cost of the electrode and can even increase the efficiency of fuel cells. Unfortunately, this type of catalysts do not currently meet the cost reduction target because they use very expensive precursors (e.g. graphene) and because the synthesis procedure involves many long and costly stages so that the price of the resulting electrocatalyst rises to values close to commercial platinum-based values.
Within the group of metal-free catalysts, carbon materials stand out. Interestingly, their surface chemistry can be modified to introduce heteroatoms, for example nitrogen, thus obtaining new materials whose behaviour is very interesting in the oxygen reduction reaction.
Although there are different methods to synthesize these materials, the carbonization of a material rich in nitrogen (for example polymers that contain nitrogen in its polymeric chain), has the advantage that the starting compound (precursor) has a defined chemical structure and it can be synthesized through different chemical processes.
There are conductive polymers with a high nitrogen content (e.g. polyaniline and polypyrrole), which are very interesting precursors because their carbonization gives rise to carbon materials with a high nitrogen content and, therefore, with active sites with higher activity to carry out the oxygen reduction reaction.
Until now, carbon materials from polyaniline obtained through different methods provide a catalytic activity and a number of electrons too low (they generate hydrogen peroxide, which is detrimental to the optimal functioning of the cell), which does not make them suitable methods for obtaining catalysts for fuel cells with low production costs.
In order to solve the technical problems described above, a low-cost and single-stage new method for the synthesis of carbon materials has been developed for their application as electrocatalysts of the oxygen reduction reaction in alkaline medium in fuel cells, or in metal-air batteries.
This is a synthesis method that allows metal-free catalysts to be obtained from a nitrogen-rich polymer (e.g. polyaniline or copolymers containing aniline in their monomer units), without the need of supports (template or sacrificial materials), or other materials that increase the cost of the product.
The synthesis procedure is based on a pre-treatment (purge) to avoid any contamination of the atmosphere, followed by a heat treatment in an inert atmosphere at temperatures above 1100ºC.
Specifically, the steps in this novel procedure are the following:
1. Introduce and distribute homogeneously the powder precursor (copolymer rich in nitrogen in which the monomer unit is aniline) in a suitable container;
2. Place the container in a hermetically sealed oven;
3. Purge the oven with an inert gas;
4. Gradually heat the oven in a controlled inert atmosphere at a certain flow rate (from room temperature to a temperature above 1100°C). The temperature in the oven is controlled by a thermocouple inserted at a point close to the centre of the container;
5. Maintain the maximum temperature constant for a certain period of time (less than one hour) in a controlled inert atmosphere;
6. Gradually cool the oven to room temperature in a controlled inert atmosphere;
7. Remove the powdered carbon material from the container. This material is characterized by the following typical composition in atomic percentage: at least 90% in carbon; between 1 and 3% in nitrogen; less than 2% in hydrogen; and between 4 and 6% in oxygen.
It is important to point out that the two key factors to be taken into account in order to obtain carbon materials that can act as electrocatalysts in the oxygen reduction reaction with high performance are:
a) Temperature treatment: the increase in temperature generates important changes in catalytic activity, reaching values in the samples obtained using the present method as high as those obtained by commercial catalysts based on platinum nanoparticles.
b) Precursor used: although catalytic activity improves with temperature increase, only polyaniline and its derivatives produce catalytic activity with values similar to commercial platinum catalysts.
By controlling these two factors, it is possible to synthesize metal-free electrocatalysts for the oxygen reduction reaction in a single-stage and in a very simple way (taking polyaniline as a precursor), which have an excellent activity towards the formation of water (without production of hydrogen peroxide), at a radically lower cost than that of a commercial platinum-based catalyst.
Carbon materials resulting from this method have a high dispersion capacity in an aqueous medium, which facilitates the formation of suspensions for later preparation of the catalysts. These suspensions remain stable over a long period of time.
Pyrolysis yield is an important parameter for an economic-industrial point of view. In this case, it is observed that the yield decreases as the temperature of the thermal treatment increases until reaching a stable value at around 1100ºC. From this temperature, the yield of polyaniline pyrolysis reaches 28% (it can be considered high compared to the pyrolysis of other precursors, whose values are less than 10%). Therefore, the synthesis of these materials using this new method is highly efficient (see Figure 1)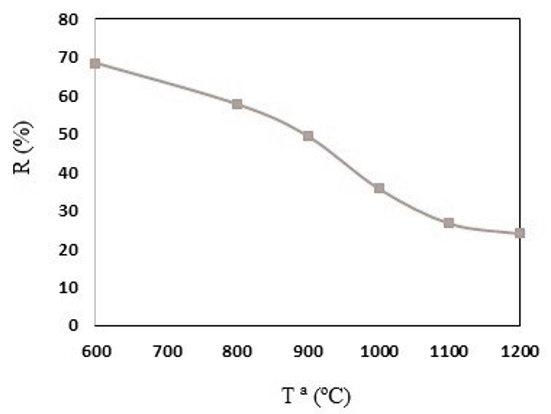
Figure 1: Plot of yield obtained in polyaniline heat treatments at different temperatures.
When a temperature above 1000ºC is used, a significant change occurs in the final carbon materials. Specifically, at 1100ºC these materials acquire acatalytic activity almost identical to commercial platinum-based catalyst (see Figure 2b), both in starting potential of the reaction and in current density limit, making themclear candidates for replacing platinum-based catalysts in fuel cells. However, an increase in the heat treatment temperature above 1100ºC does not lead to an improvement in the catalytic activity of the synthesized material.
Regarding the number of electrons transferred, a factor related to hydrogen peroxide performance (see Figure 2a), hydrogen peroxide values below 5% have been measured in the useful working range of fuel cells (i.e. between 0.6 and 1.0 vs RHE), indicating that it prevents formation of by-products detrimental to the life-time of fuel cells.
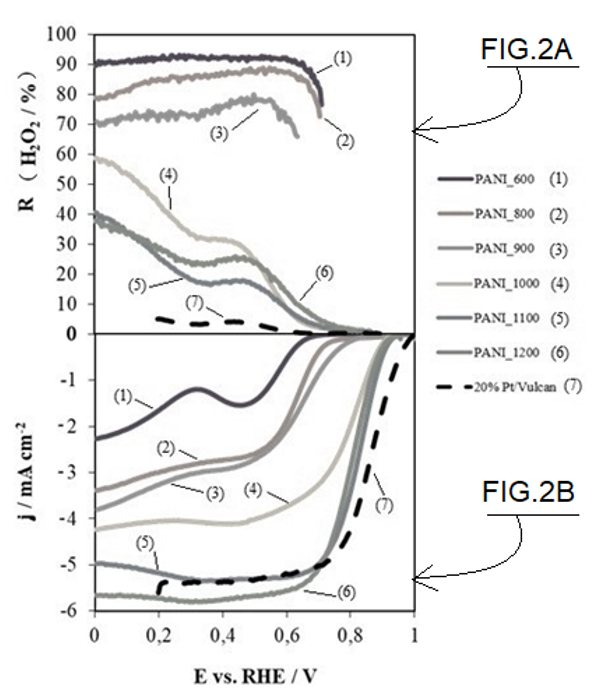
Figure 2: (a) hydrogen peroxide yield; (b) linear scan voltammetry curves for carbon materials in 0.1 M potassium hydroxide solution saturated in oxygen at 5 mV.s-1 and 1600 rpm.
Stability was studied, and a clear improvement in the stability of the synthesized material (95%) with respect to a commercial platinum-based catalyst (87%) was observed. Moreover, itsresistance to methanol poisoning has also been studied, and it has been possible to conclude that, after adding methanol up to a concentration of 1 M, the synthesized catalyst works without losing catalytic activity, while commercial platinum-based catalyst was quickly poisoned and lost its catalytic activity in the presence of methanol (see Figure 3).
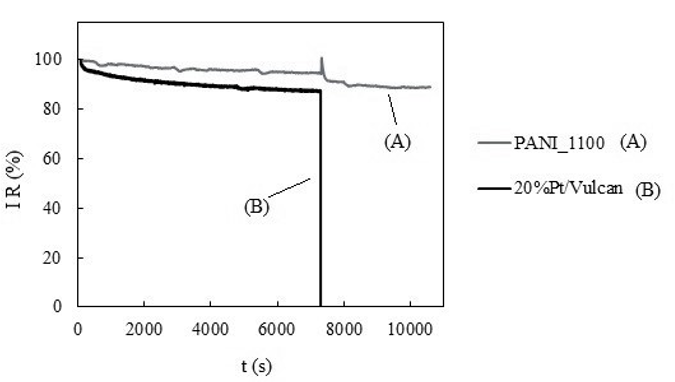
Figure 3: stability and methanol poisoning study of the sample treated at 1100°C in a nitrogen atmosphere (A) and the commercial platinum-based catalyst (B).
Instead of polyaniline, other copolymers containing aniline can be used as precursors (aniline being a determining monomer to obtain excellent catalytic activity) (see Figure 4). However, with these copolymers, pyrolysis yield is lower than those obtained with polyaniline, making itthe most viable and effective precursor with respect to the use of other monomers.
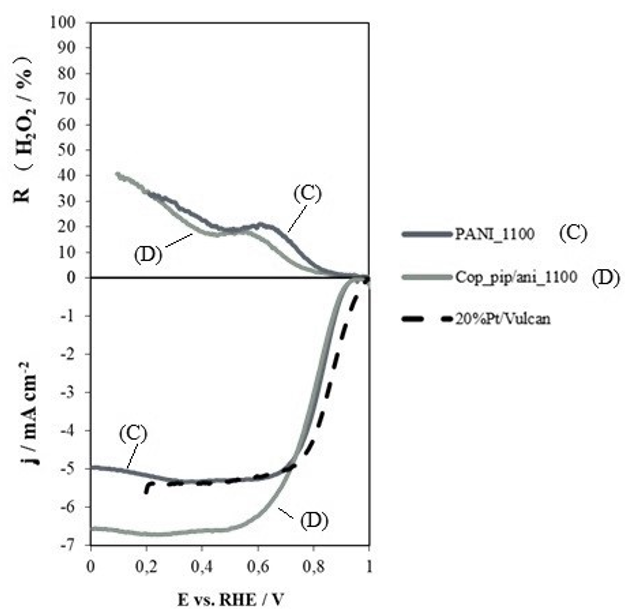
Figure 4: hydrogen peroxide yield and linear scanning voltammetry curves for heat-treated polyaniline at 1100ºC (C) and a copolymer based on piperazine and aniline (D) in a 5:1 ratio, in 0.1 M potassium hydroxide solution saturated in oxygen at 5 mV.s-1 and 1600 rpm.
Regarding the use of different inert atmospheres (argon, nitrogen, etc.) during the synthesis procedure, it can be concluded that theexcellent catalytic activity of the carbon materials obtained by this method is independent of the atmosphere used (see Figure 5).
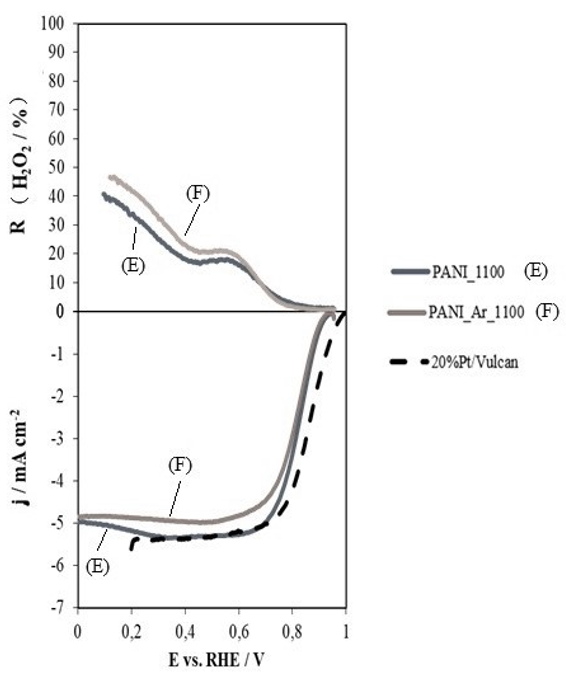
Figure 5: (a) hydrogen peroxide performance; (b) linear scan voltammetry curves for polyaniline thermally treated at 1100ºC in nitrogen (E) and polyaniline treated at 1100ºC in argon (F) atmosphere, in 0.1 M potassium hydroxide solution saturated in oxygen at 5 mV.s-1 and 1600 rpm.
Finally, it should be underlined that themass/flow ratio does not modify the final pyrolysis yield, nor does it affect the final catalytic activity of the materials synthesized by this method (see Figure 6).
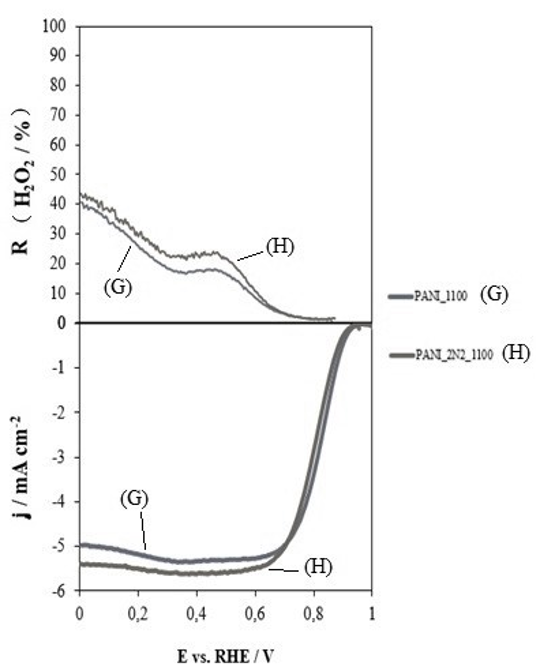
Figure 6: (a) performance in hydrogen peroxide; (b) linear scan voltammetry curves for heat-treated polyaniline at 1100ºC in nitrogen with a flow rate of 100mL/min (G) and polyaniline treated at 1100ºC in the same atmosphere with a flow rate of 200mL/min (H), in 0.1 M potassium hydroxide solution saturated in oxygen at 5 mV.s-1 and 1600 rpm.
ADVANTAGES OF THE TECHNOLOGY
The main advantages of this novel method are listed below:
10. The oxygen reduction reaction has a high selectivity, since it is carried out through a mechanism of four electrons whose final reaction product is water (with a minimum production of reaction intermediaries, less than 5%, which could damage the fuel cell).
11. They are environmentally friendly materials, since they are metal-free catalysts.
12. They can be used as electrodes in fuel cells and metal-air batteries.
13. They reduce the total cost of the fuel cell.
14. These are major candidates to replace existing commercial platinum-based catalysts in alkaline medium.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
Although thermal treatment of polymers containing aniline in their monomer units is known within the state of the art, the present method differs from the other procedures in the following aspects:
CURRENT STATE OF THE TECHNOLOGY
These novel electrocatalysts have been successfully synthesized at laboratory scale using the method described above.
Kinetic parameters obtained for these new electrocatalysts, whose values are similar to those obtained by commercial platinum-based catalysts, are listed below:
APPLICATION MARKETS
This invention is framed in the energy sector, specifically in the area related to chemical transformations derived from the transfer of electrons produced in electrocatalysts.
This technology makes it possible to obtain metal-free carbon materials for application as excellent electrocatalysts (cathode) in the oxygen reduction reaction under alkaline conditions in hydrogen or methanol low temperature fuel cells, or in metal-air batteries.
Therefore, this technology finds its application in the following industrial sectors:
COLLABORATION SOUGHT
We are looking for companies interested in acquiring this technology for commercial exploitation through:
Company profile sought:
Ahead of the current Coronavirus outbreak, Innoget is fully committed to contributing to mobilizing scientific and expert communities to find a real solution to the Covid-19 pandemic. Therefore, we're supporting worldwide calls and programs that could help in any aspects of the coronavirus crisis.
Is your organization promoting or looking for innovation or research initiatives to mitigate the Covid-19 outbreak? Email us at covid19@innoget.com to list them.
Channeled through Innoget's online open innovation network, initiatives in the health, virology, medicine, or novel technologies applied to human health, among others, are listed and disseminated to Innoget members -ranging from hospitals, research institutes, scientists, businesses, and public administrations- and innovation partners worldwide.
Create your free account to connect with Javier Montiel Bonmatí and thousands of other innovative organizations and professionals worldwide
Send a request for information
to Javier
Technology Offers on Innoget are directly posted
and managed by its members as well as evaluation of requests for information. Innoget is the trusted open innovation and science network aimed at directly connect industry needs with professionals online.
Need help requesting additional information or have questions regarding this Technology Offer?
Contact Innoget support