- RAMOT at Tel Aviv University Ltd.
- From Israel
- Responsive
- Innovative Products and Technologies
Summary of the technology
Rhenium (Re) exhibits a unique combination of properties qualifying it to be a high performance engineering material, for applications in aerospace, electronics, medical devices and fuel cells. Rhenium has the second highest melting point of all metals, the third highest Young’s modulus of elasticity and the fourth highest density. While the other refractory metals have a body centered cubic (bcc) structure, Re has a hexagonal close packed (hcp) structure. Consequently, it does not possess a ductile-to-brittle transition and, therefore, can safely be used at subzero temperatures. In addition Re has a creep-rupture strength over a wide high temperature range, up to about 2000°C. These properties imply that structures made of Re have excellent mechanical stability and rigidity, enabling the design of parts with thin sections, extremely attractive for high-temperature structural and high energy system applications. Re nanostructures are likely to exhibit remarkable advantages over other nanostructured metals.
Project ID : 3-2012-389
Details of the Technology Offer
The Technology
We present low-cost, efficient, long life-time nanostructured Re-alloy coatings for catalyzing low temperature dry reforming of methane (DRM).
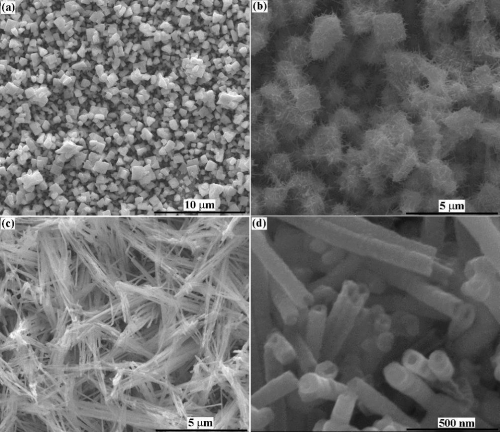
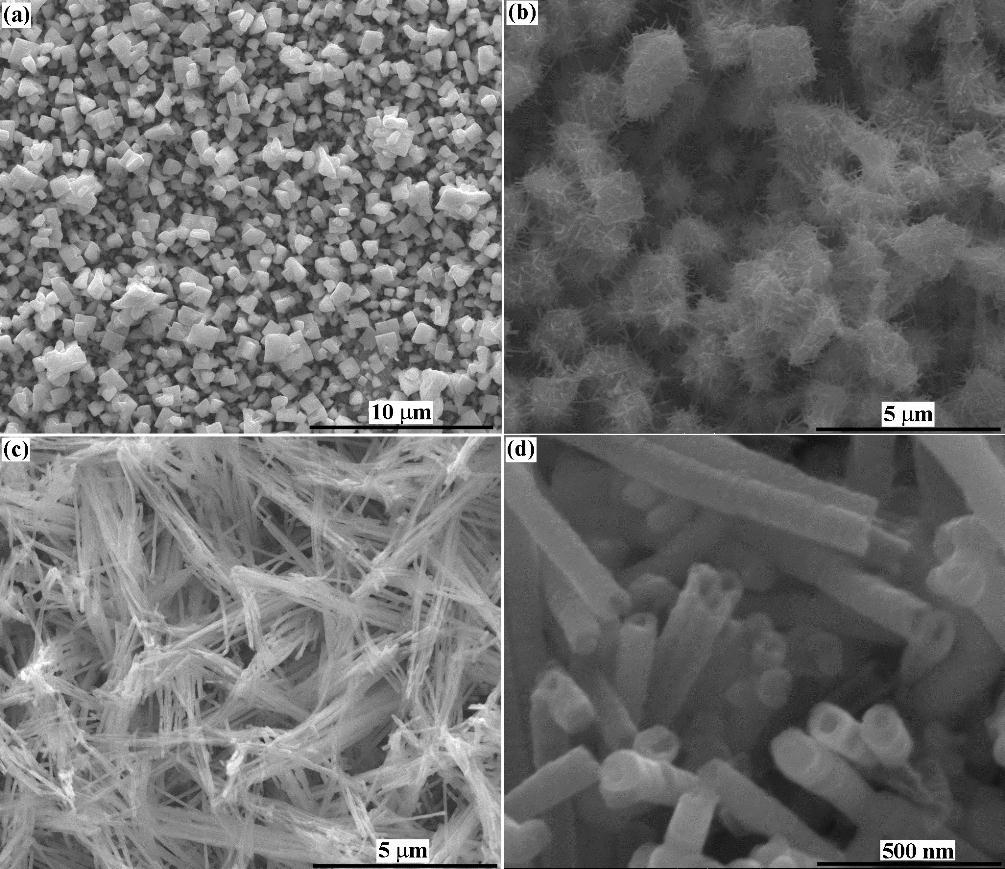
Figure: SEM secondary electron image from Re-SN nanostructure coating on copper and high magnification of this image.
Nickel, cobalt, and iron are used as standard catalysts in DRM, however, they are subject to rapid deactivation through coking. As a result, the reaction temperature for DRM routinely exceeds 800°C to avoid the coke formation. Rhenium has also been shown to be active towards DRM with the added benefit of being more resistant to coking. The inclusion of rhenium atoms into catalyst films can therefore improve the activity and lifetime of the catalyst, and open the door for low temperature operation.
We are forming nanostructured Re-Me (Me = Ni, Co, Fe, Sn) catalyst films to support the DRM at low temperatures and minimal catalyst deactivation. Additionally, we wish to exploit the nanostructure of the films to improve reactivity. For example, rhenium alloy nanowire films developed in our lab can allow for a more efficient heat transfer to the reaction zone, leading to higher conversion and slower poisoning. The implementation of the DRM reaction at low temperatures (650°C) can save energy companies billions of dollars by lowering operational costs, and thus providing a route to convert methane to its liquid form in an economical manner.
The Need
The worldwide crude oil supply will deplete in approximately 45 years if we continue at our current rate of consumption. By comparison, the world’s natural gas (80-99% methane) supply is predicted to be large enough to supply our energy needs for the next millennium.
Gas-to-liquid (GTL) technology is a sequence of chemical reactions that converts methane into liquid fuels, a conversion required for its safe and economic transportation from remote locations. The first of these reactions is the dry reforming of methane (DRM). Due to the endothermic nature of DRM, temperatures upwards of 800°C are generally required to achieve high methane conversion with minimal catalyst poisoning. At low temperatures, however, catalyst poisoning through coking is favored and methane conversion is low due to the high activation barrier.
Project Status
The processes of Re-Sn and other Re alloy nanowire formations has been established and is well controlled. Through careful selection of the alloying metal (namely Ni, Co, and Fe) and its concentration, the faradaic efficiency of Re-alloy films can be as high as 96%. The rhenium content of the film can range between 0 and 95%, and the growth rate of the film can range between 10 and 25 μm/h. Planning the use of pulse plating in order to control the
composition and structure of the deposited alloy film is also underway.
Patents
US Patent Application pending.
Supporting Publications
Naor-Pomerantz, A., N. Eliaz, and E. Gileadi, Electrodeposition of rhenium–tin nanowires.
Electrochimica Acta, 2011. 56(18): p. 6361-6370.
Naor, A., et al., Direct Experimental Support for the Catalytic Effect of Iron-Group Metals on
Electrodeposition of Rhenium. Electrochemical and Solid-State Letters, 2010. 13(12): p. D91-D93.