Showing 1 to 15 of 2075 results


Potyvirus viral detection kit
Patents for licensing Consejo Superior de Investigaciones Científicas

Sistema de detección de intrusiones basado en agentes
Research Services and Capabilities UNIVERSIDAD DE BURGOS

Chinese teaching method for blind people
Patents for licensing UNIVERSIDAD DE BURGOS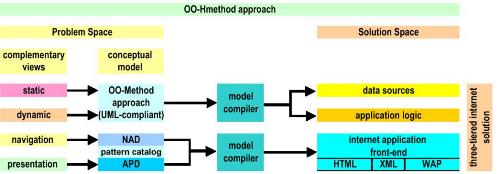

Software tool and method for modelling device-independent web applications
Patents for licensing Universidad de Alicante

Waste Treatment & Industrial Valorization
Research Services and Capabilities FUNDITEC

Control method for a neuroprosthetic device for the reduction of pathological
Patents for licensing CSIC - Consejo Superior de Investigaciones Científicas

A functional salt substitute derived from grape pomace with preservative effects and its manufacturing method
Patents for licensing UNIVERSIDAD DE BURGOS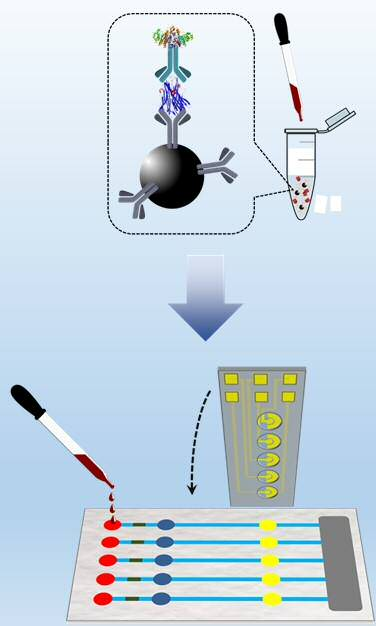

Point-of-care analytical device for rapid and multiplexed detection of biomarkers
Patents for licensing Consejo Superior de Investigaciones Científicas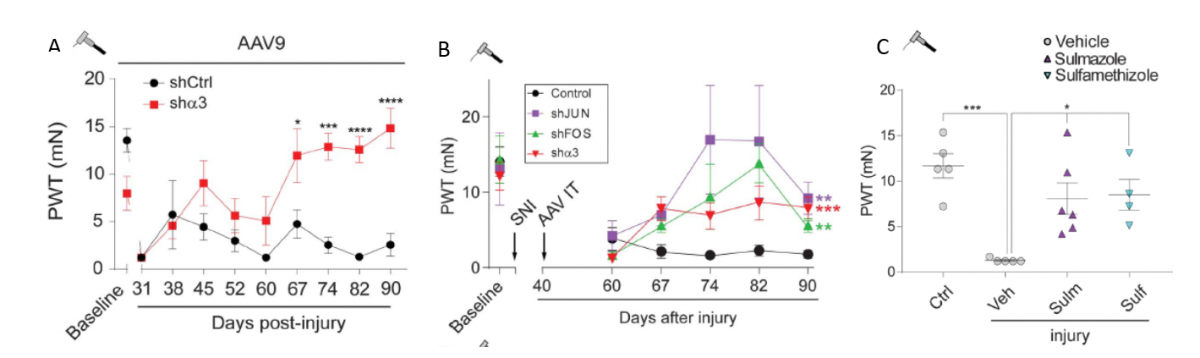

Treating Chronic Pain by Inhibiting Importin Alpha 3
Patents for licensing Yeda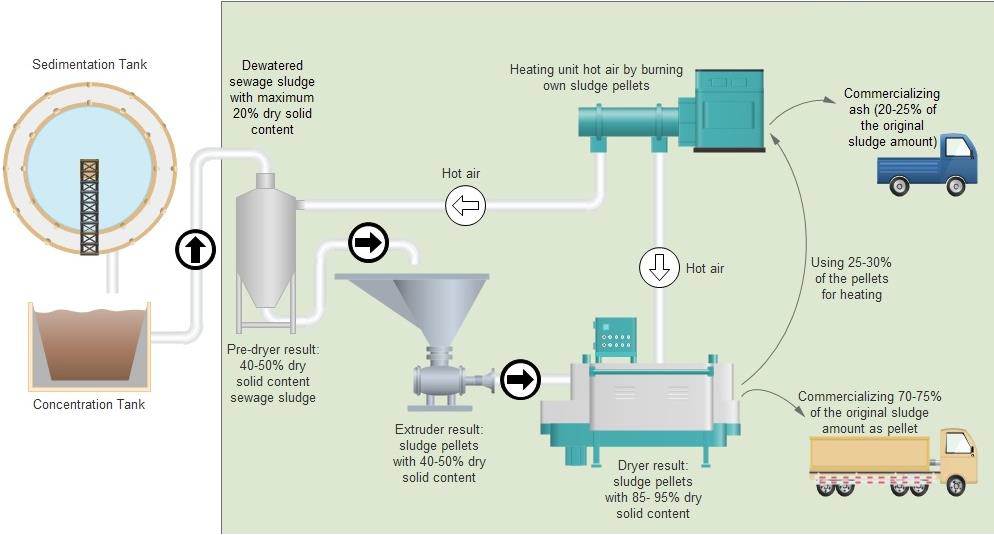

Innovative Technology to Utilize and Recycle Sewage Sludge
Innovative Products and Technologies Laser Consult Ltd.![A new packaging system for a real PICK AND PACK solution dedicated to warehouse logistics (e-commerce, automated picking order lin[…]](https://static3.innoget.com/uploads//a4b911ea7998fd0fa100a3cb2d882798c88f168c.jpg)

A new packaging system for a real PICK AND PACK solution dedicated to warehouse logistics (e-commerce, automated picking order lin[…]
Innovative Products and Technologies INAWA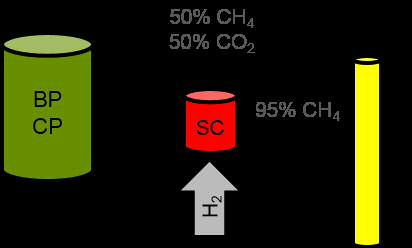

Catalyst for the upgrading of raw biogas
Patents for licensing Consejo Superior de Investigaciones Científicas

New compound for body weight regulation with applications in animal breeding
Patents for licensing Consejo Superior de Investigaciones Científicas

Equipment For The Determination Of The Isobaric Vapour-Liquid-Solid And Vapour-Liquid-Liquid-Solid Equilibrium
Patents for licensing Universidad de Alicante

