Showing 1 to 15 of 2075 results
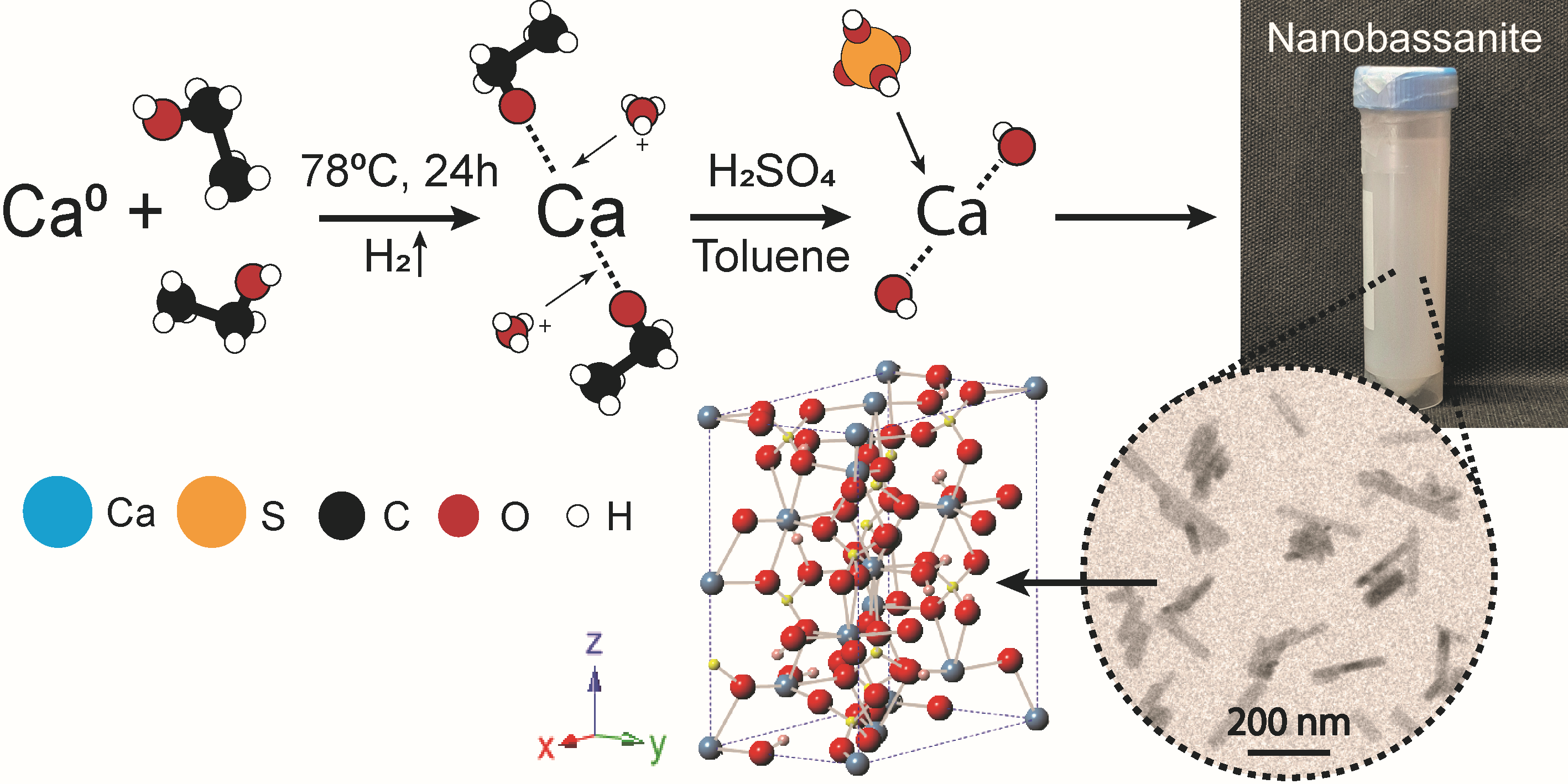

Novel bassanite nanoparticles production method
Patents for licensing Universidad de Granada

Biofeedback system for urinary incontinence treatment
Patents for licensing Universitat Politècnica de Catalunya - UPC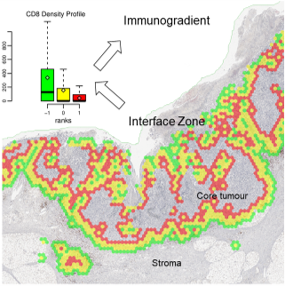

Automated Tumour-Stroma Interface Zone Detection for Anti-Tumour Response Assessment by Immunogradient Indicators
Knowhow and Research output Vilnius University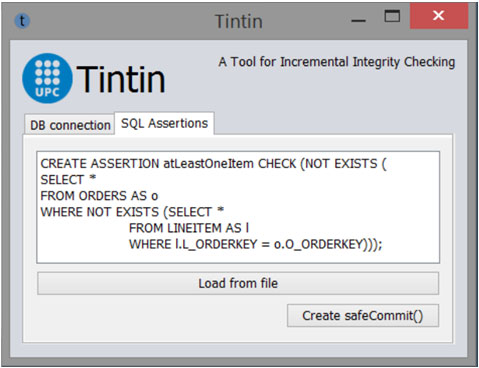

TINTIN: A Tool for INcremental INTegrity checking
Patents for licensing Universitat Politècnica de Catalunya - UPC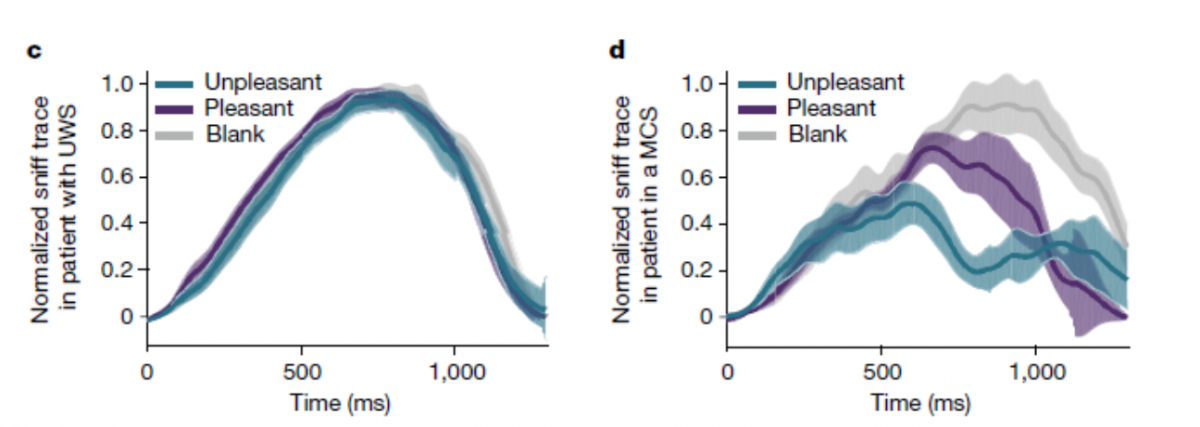

A Marker for Consciousness State Following Brain Injury
Patents for licensing Yeda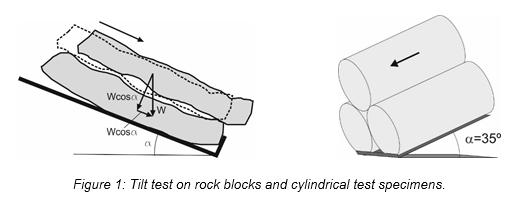

New automated machine for rock tilt tests
Patents for licensing Universidad de Alicante

METHOD FOR THE PREDICTION OR PROGNOSIS OF DEVELOPING RHEUMATOID ARTHRITIS
Patents for licensing Universidad de Málaga

Wireless Device and Topologies for Energy Harvesting Based on Maxwell Displacement Current
Innovative Products and Technologies Hub APTA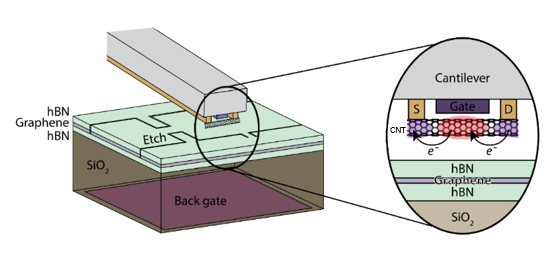

CNT-Based Structure for High-Resolution Semiconductor Process Control
Patents for licensing Yeda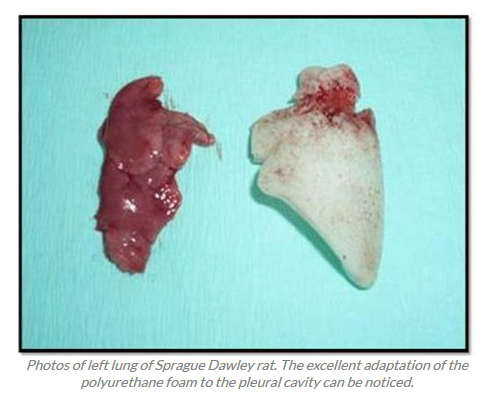

Novel self-expanding polyurethane foams intended for inert filling of pleural and other human cavities.
Patents for licensing UNIVERSIDAD DE ALICANTE

Novel KRAS G12C agent with improved potency & PK properties available
Innovative Products and Technologies ShamRock Pharmaceuticals

Syrupy products powder preparation method
Patents for licensing UNIVERSIDAD DE BURGOS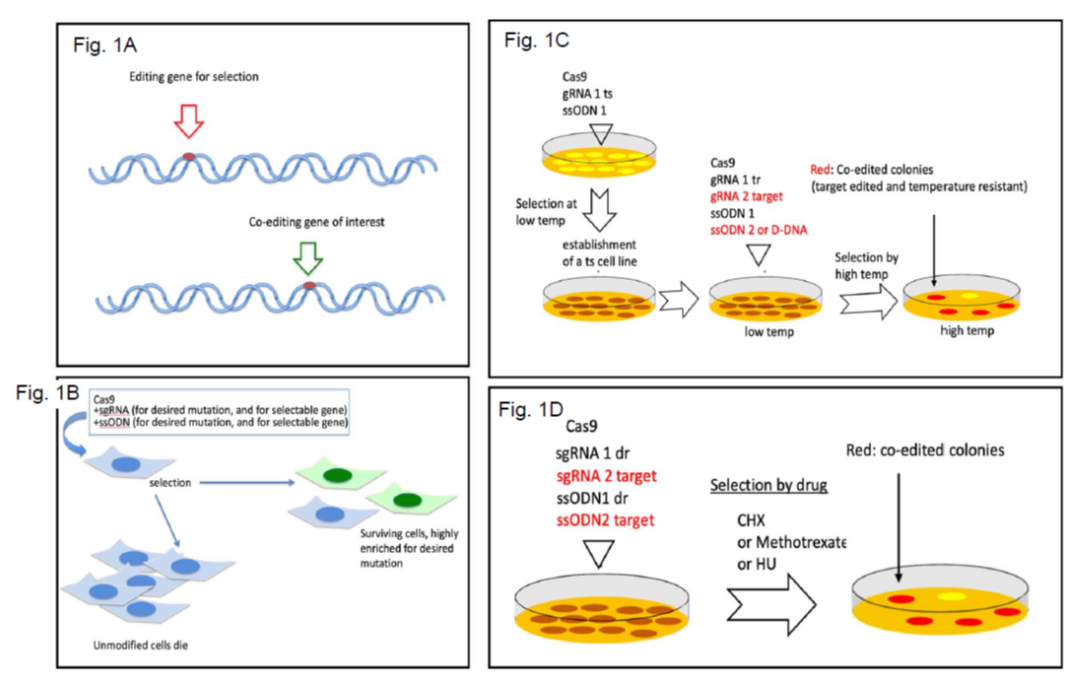

Scarless CRISPR-mediated Genome Editing
Patents for licensing Yeda

Method for determining prognosis in subjects diagnosed with pulmonary arterial hypertension
Patents for licensing CINBIO

