Showing 1 to 15 of 2075 results
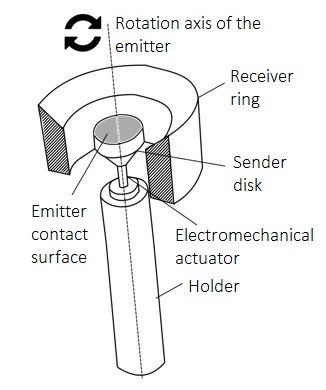

Device for measuring the biomechanical properties of the cornea
Patents for licensing Universidad de Granada

Rice husk as a filter for the removal of contaminants in water
Patents for licensing Consejo Superior de Investigaciones Científicas

Automatic component disassembling for dismantling and recycling systems.
Patents for licensing Universidad de Alicante

Prize-winning KIERSOL Technology - Alkali Carbonate based absorbent for removing carbon dioxide
Innovative Products and Technologies Korea Institute of Energy Research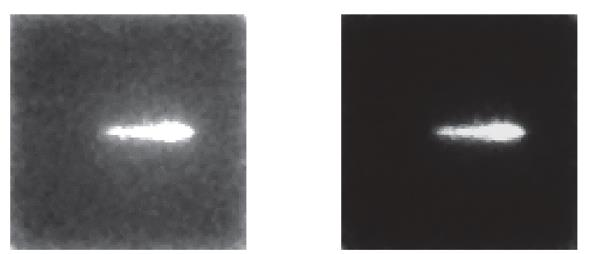

Improvement of the signal / noise ratio in detectors used in hadronic therapy
Patents for licensing CSIC - Consejo Superior de Investigaciones Científicas

LIH383: novel modulator of the opioid system regulator ACKR3
Patents for licensing Luxembourg Institute of Health

Label Free Detection and Imaging of Biofilms
Knowhow and Research output National Biofilms Innovation Centre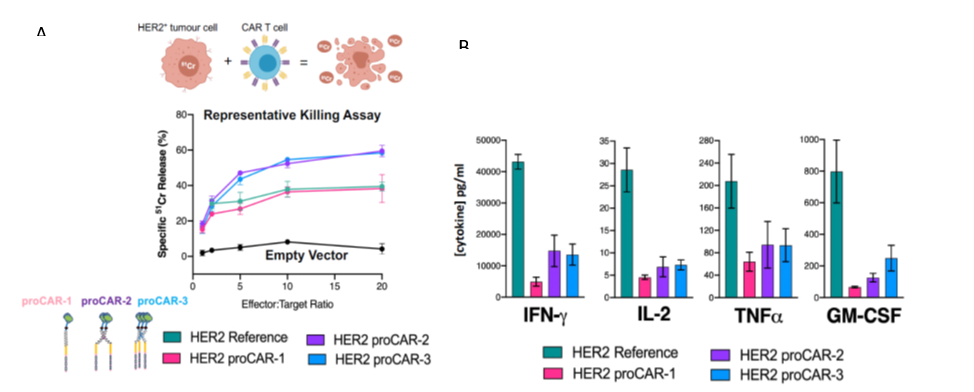

Safer CAR-T cell platform
Patents for licensing Yeda

New tannin-rich extract from vegetal sources for tanning hides and skins
Patents for licensing Universitat de Lleida![A new packaging system for a real PICK AND PACK solution dedicated to warehouse logistics (e-commerce, automated picking order lin[…]](https://static3.innoget.com/uploads//a4b911ea7998fd0fa100a3cb2d882798c88f168c.jpg)

A new packaging system for a real PICK AND PACK solution dedicated to warehouse logistics (e-commerce, automated picking order lin[…]
Innovative Products and Technologies INAWA![RNA Targeted Library for post transcriptional gene regulation researches, anticancer, antiviral and antibacterial drug discovery […]](https://static1.innoget.com/uploads//a00f99d109d7028322bc0729ae707cb3e8afc5ec.jpg)

RNA Targeted Library for post transcriptional gene regulation researches, anticancer, antiviral and antibacterial drug discovery […]
Research Services and Capabilities Otava Research Institute

Apparatus for producing silicon nanocrystals based on inductively coupled plasma.
Innovative Products and Technologies Korea Institute of Energy Research

Histone Deacetylases (HDAC) Targeted Libraries. Screening compounds for epigenetic drug discovery.
Innovative Products and Technologies Otava Research Institute

Recycling of precious metals via an efficient process with no toxic byproduct
Patents for licensing Yeda

