Showing 1 to 15 of 2075 results


Immunoassay for detection of Pseudomonas aeruginosa Quorum Sensing molecules
Patents for licensing Consejo Superior de Investigaciones Científicas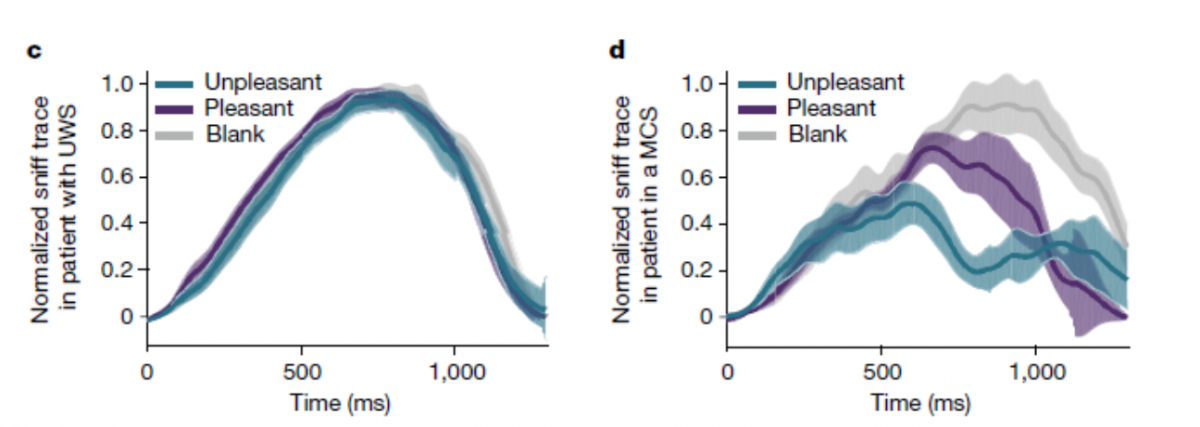

A Marker for Consciousness State Following Brain Injury
Patents for licensing Yeda

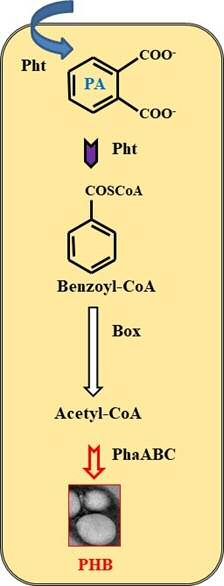
Production of isophorone derivatives by fungal peroxygenases
Patents for licensing Consejo Superior de Investigaciones Científicas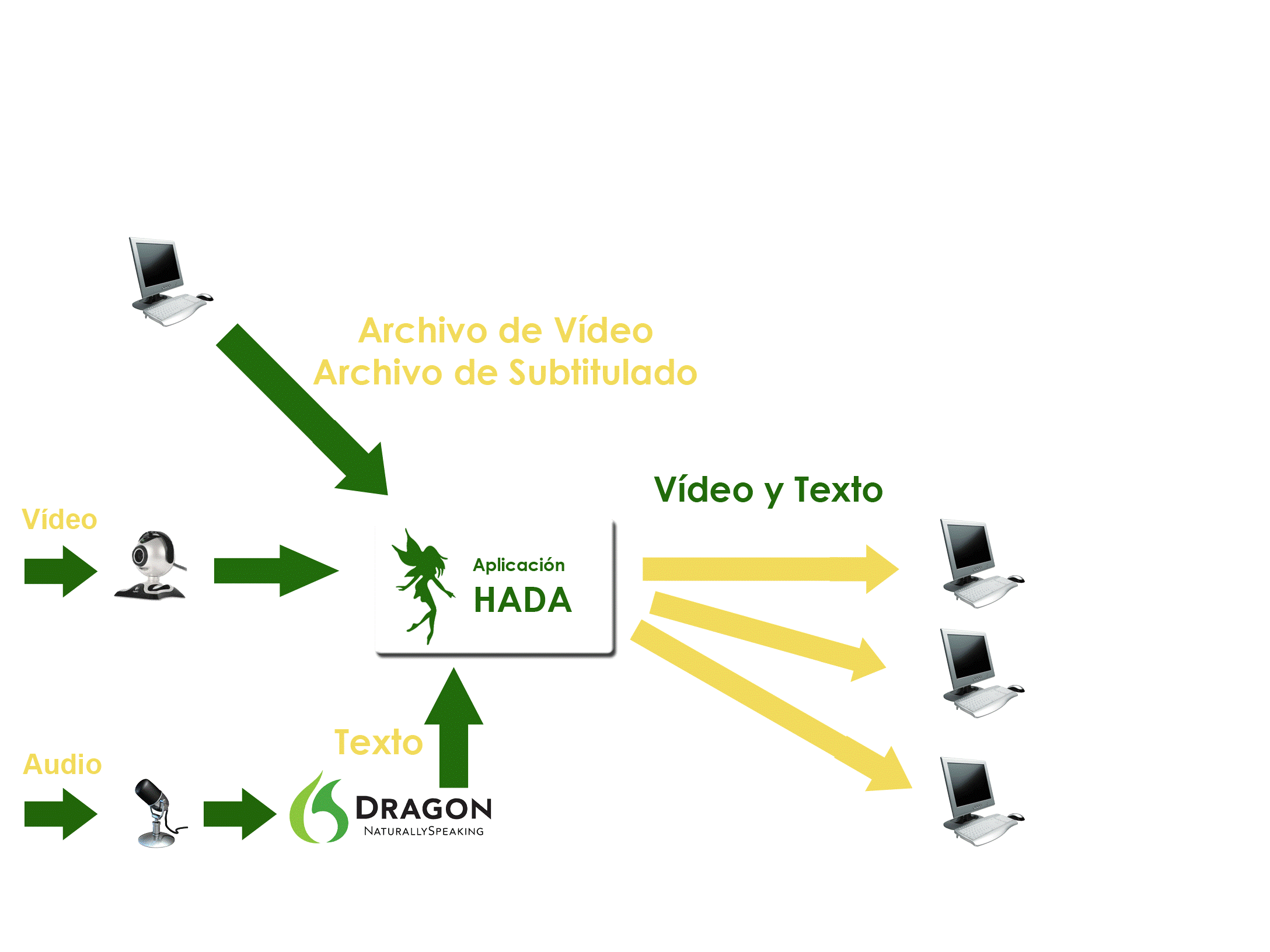

HADA (Hearing Impaired Assistance Tool). Real-time text and video streaming for the hearing impaired people
Patents for licensing UNIVERSIDAD DE BURGOS

Method for determining prognosis in subjects diagnosed with pulmonary arterial hypertension
Patents for licensing CINBIO

High resolution paterning in organic semiconductors
Patents for licensing Consejo Superior de Investigaciones Científicas

Hospital emissions: N2O abatement
Innovative Products and Technologies Centre Technology Transfer CITTRU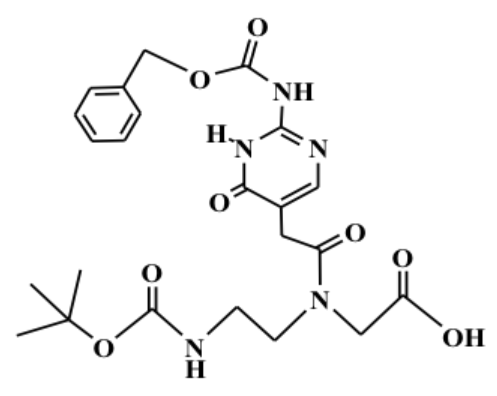

Boc-J(Z)-Aeg-OH Cas 163081-03-6
Innovative Products and Technologies Creative Peptides

Catalyst for nitrous oxide (N2O) decomposition
Patents for licensing Consejo Superior de Investigaciones Científicas

Vertical axis wind turbine for slow and changing wind speed
Innovative Products and Technologies Laser Consult Ltd.

Synthesis of Arylpiperazines as Perspective Toxoplasma gondii Dihydrofolate Reductase Inhibitors
Innovative Products and Technologies Ukrainian Laboratories![New methods for the detection and discrimination of contaminants and organic metabolites of high environmental impact by means of […]](https://static9.innoget.com/uploads//79d0a575a1a9ecbca8a1f563aaae257a93d651a5.png)

New methods for the detection and discrimination of contaminants and organic metabolites of high environmental impact by means of […]
Research Services and Capabilities UNIVERSIDAD DE BURGOS

Observatory for Climate, Environment and Biodiversity
Research Services and Capabilities Luxembourg Institute of Science and Technology (LIST)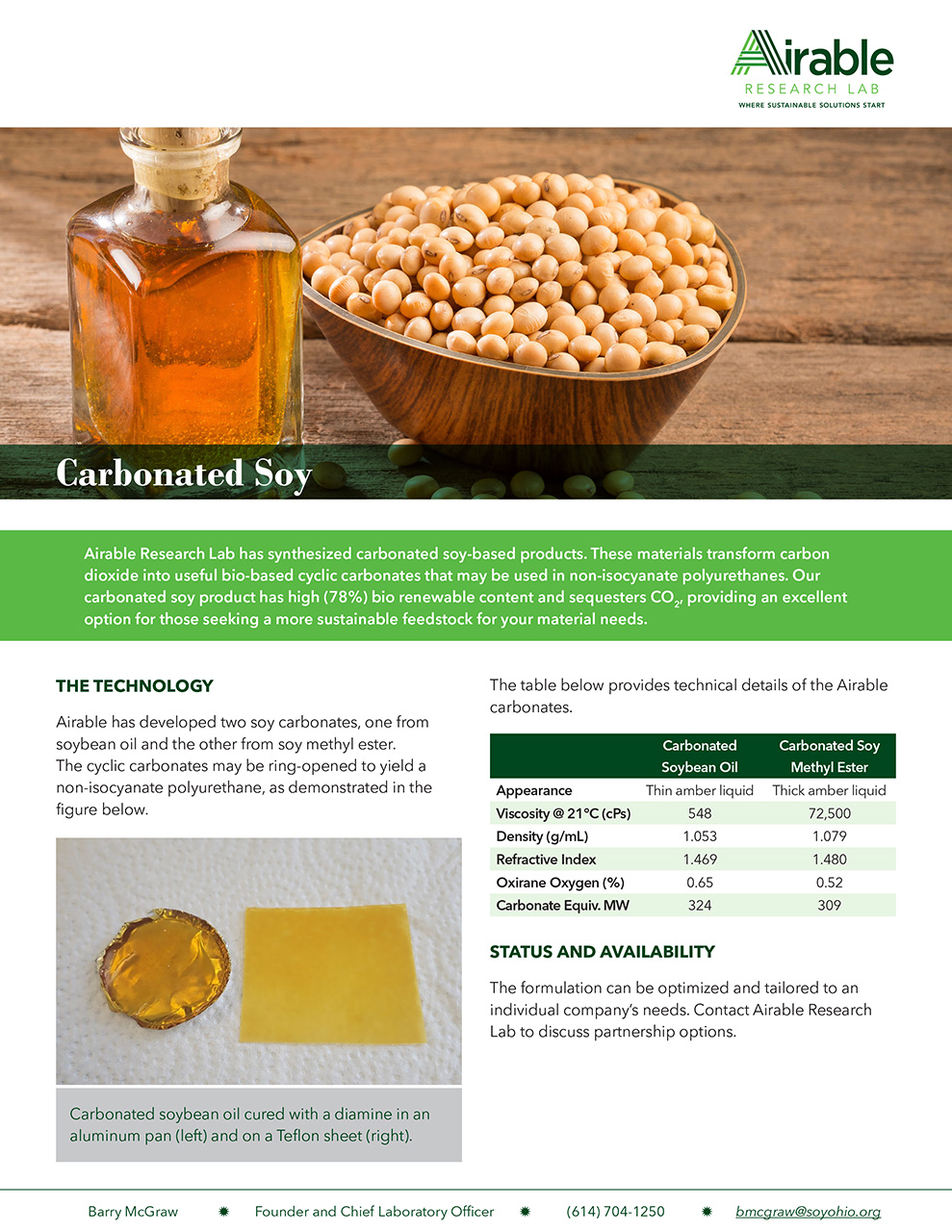

Carbonated Soy-Based Products That Transform Carbon Dioxide
Innovative Products and Technologies Airable Research Lab, business line of Ohio Soybean Council