Showing 1 to 15 of 2075 results

Cell-free Expression System
Research Services and Capabilities Leading Biology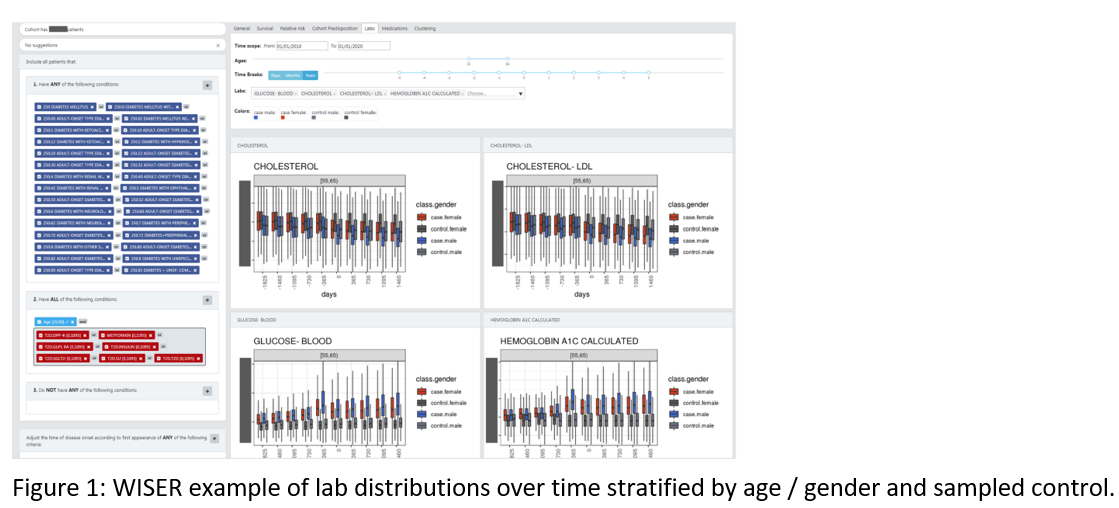

WISER – A Holistic Analysis Of Large Scale Electronics Health Records
Patents for licensing Yeda

New Improved Plant Protein Upcycled from Rice Brokens
Innovative Products and Technologies Incr-Edible Ingredients

MicroC@MPUS® . A Web-based educational environment
Patents for licensing Universidad de Alicante

Active Masks with Virus Neutralizing Properties
Innovative Products and Technologies Covid-19 Innovation Challenges by Innoget

Soy-Based Odorant for Carbon Dioxide Pipelines
Innovative Products and Technologies Airable Research Lab, business line of Ohio Soybean Council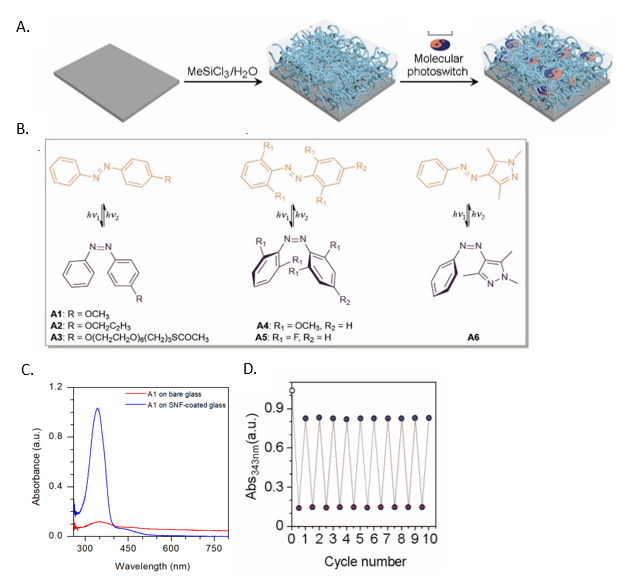

Reversible Operation of Molecular Switches on Solid Surfaces
Patents for licensing Yeda

A resilient and user-friendly CAPTCHA
Patents for licensing Unismart - University of Padua Foundation

New Antiviral Drugs from Bacterial Natural Products
Patents for licensing Yeda

Systematic Computational Drug Repositioning to Treat COVID-19
Innovative Products and Technologies Covid-19 Innovation Challenges by Innoget

US9226722 (B2) - Medical Imaging System and Method with Separate Primary and Scattered Components
Patents for licensing Syncoda Technologies

SerpinB3 inhibitor for tumor treatment
Patents for licensing Unismart - University of Padua Foundation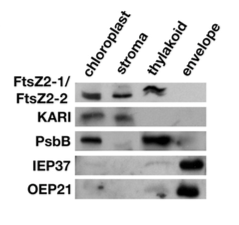

Polyclonal Antibody Serum Preparation
Research Services and Capabilities Leading Biology

Antibody Drug Conjugates (ADCs)
Innovative Products and Technologies ALL Chemistry Inc.