Showing 1 to 15 of 2075 results

Pressure pulsation and vibration damping in reciprocating compressors manifold
Knowhow and Research output Cracow University of Technology

Electrochemical sensor for detecting water pollutants
Patents for licensing ICMAB-CSIC

mynd, a powerful AI-driven text mining platform
Innovative Products and Technologies mynd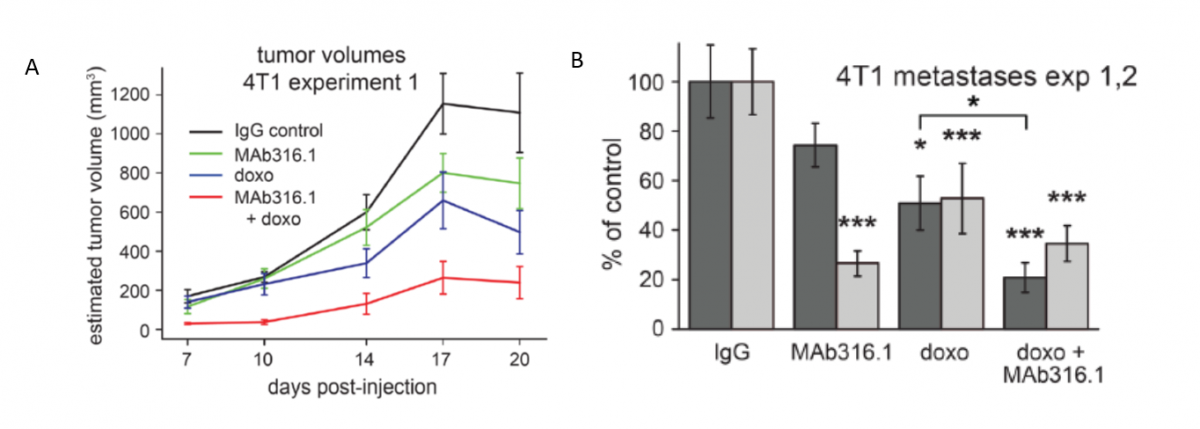

Anti-QSOX1 Antibody for Treating Cancer and Metastasis
Patents for licensing Yeda

Producing bioethanol and/or glucose syrups from raw starch
Patents for licensing Unismart - University of Padua Foundation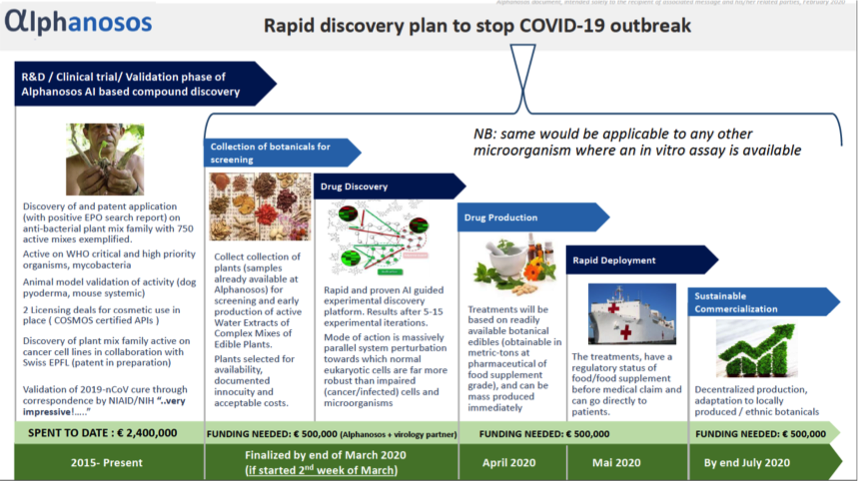

Alphanosos rapid discovery technology against COVID 19
Innovative Products and Technologies Alphanosos

Demetalization of sewage sludges through physico-chemical processes
Research Services and Capabilities UNIVERSIDAD DE BURGOS

Preserveing the value of food and agriculture commodity .
Innovative Products and Technologies NuVive , LLC

Poly divinylbenzene for polypeptide synthesis
Patents for licensing Unismart - University of Padua Foundation

Removable and reconfigurable system for corner connection of square tube profiles
Patents for licensing University of Vigo

GEO-MOBILITY: Application that helps people with disabilities or reduced mobility to control their environment
Patents for licensing UNIVERSIDAD DE BURGOS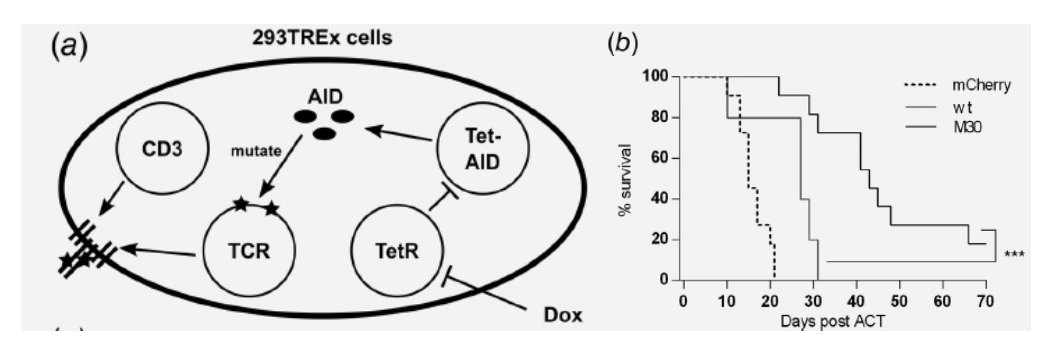

Optimizing T Cell Receptor Avidity for Adoptive T Cell Therapies
Patents for licensing Yeda
Direct Solar Lighting Technology
Investment Opportunities in Startups and Spinoffs i-EL Technologies

New vaccine candidate against SARS-CoV-2 based on MVA vector
Patents for licensing CSIC - Consejo Superior de Investigaciones Científicas

