Showing 1 to 15 of 2075 results
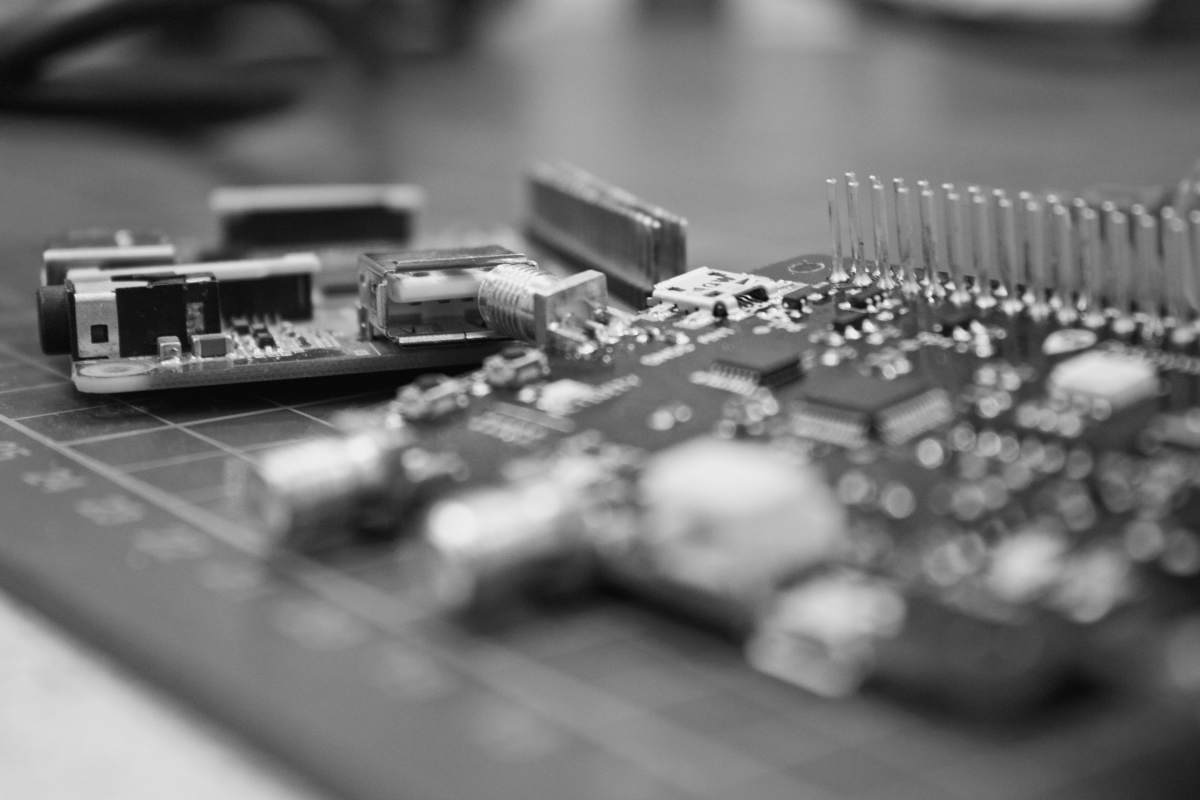

PoE led driver : a versatile power over ethernet LED driver for entertainment and light control
Innovative Products and Technologies Creaspin
Superior Battery Grid Technology
Patents for licensing i-EL Technologies

Performance management of industrial assets using a digital twin platform
Innovative Products and Technologies Cardiff University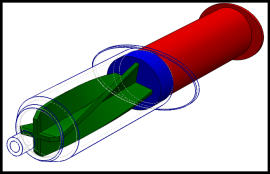

Novel syringes and concepts for homogeneous injection of bone cements
Patents for licensing Universitat Politècnica de Catalunya - UPC

Interactive automatic gate to help people with some kind of disability.
Innovative Products and Technologies BATHINNOVAX

Knowledge on occupational safety and health (OSH)
Knowhow and Research output PEROSH

New Antiviral Drugs from Bacterial Natural Products
Patents for licensing Yeda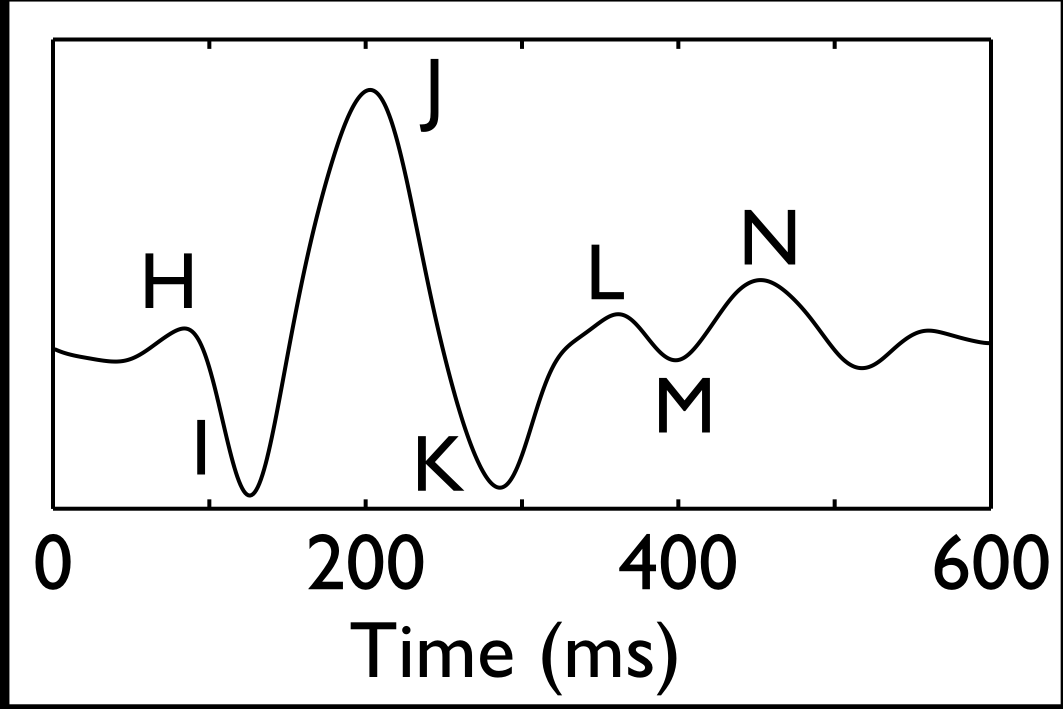

Cardiovascular health assessment from a common electronic weighing scale
Patents for licensing Universitat Politècnica de Catalunya - UPC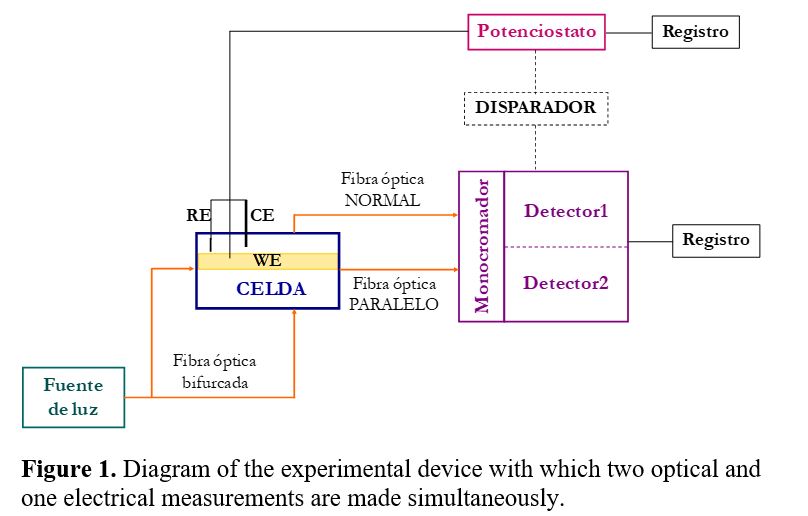

Devices for analysis and characterization of materials using electrochemical-based multiple response techniques
Research Services and Capabilities UNIVERSIDAD DE BURGOS

Research and development into new fertilizers and biostimulants
Knowhow and Research output Universidad de Alicante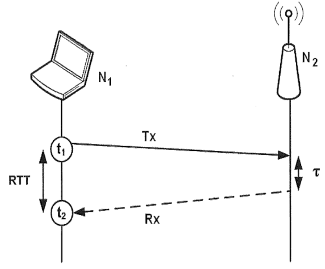

Process and system for calculating distances between wireless nodes
Patents for licensing Universitat Politècnica de Catalunya - UPC

The use of microorganisms in the plant’s production
Patents for licensing Jagiellonian University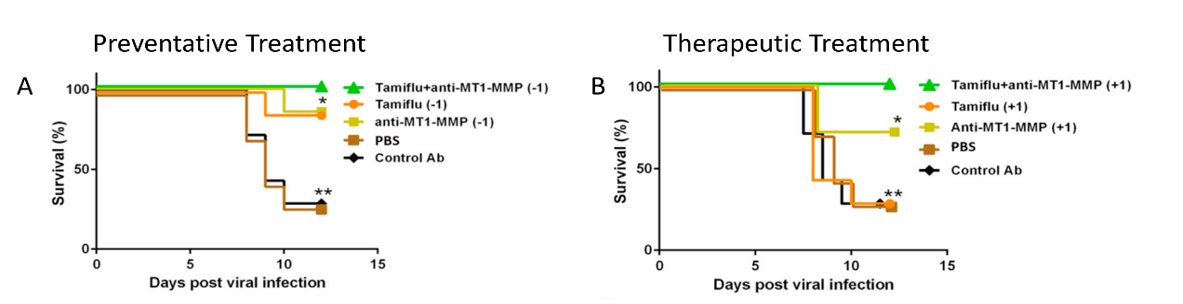

Antibody for Preventing/Treating Secondary Respiratory Infections
Patents for licensing Yeda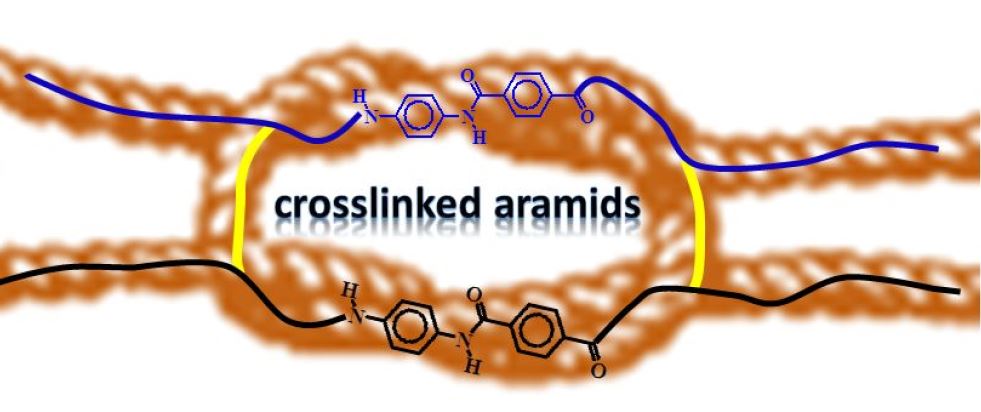

Crosslinking aromatic polyamides (aramids)
Patents for licensing UNIVERSIDAD DE BURGOS

