Showing 1 to 15 of 2075 results
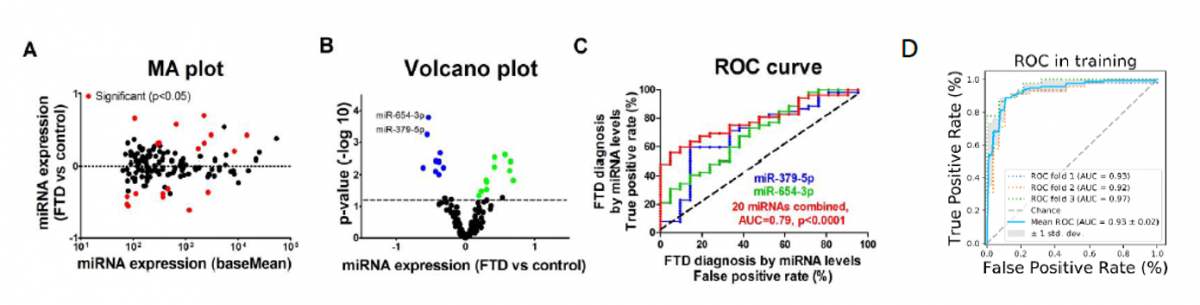

Frontotemporal Dementia Diagnosis Using Circulating microRNA Biomarkers
Patents for licensing Yeda

Biomarkers for the diagnosis of pediatric B-cell acute lymphoblastic leukemia (B-ALL).
Patents for licensing Universidad de Granada

Know-how in domotics and intelligent environments
Patents for licensing Universidad de Alicante

Equipment for planting woody-seedlings in areas of precipitation deficiency
Patents for licensing Czech University of Life Sciences

Ink for the production of superconducting flexible tapes
Patents for licensing ICMAB-CSIC

Reusable Gold Nanostars Substrates for Signal Amplification for Diagnostics
Patents for licensing Yeda

Device authentication through earphones
Patents for licensing Unismart - University of Padua Foundation

Protein Based Treatment for Duchene Muscular Dystrophy and Cancer
Patents for licensing Yeda

Special Functional Antibody Service
Research Services and Capabilities Leading Biology

US9226722 (B2) - Medical Imaging System and Method with Separate Primary and Scattered Components
Patents for licensing Syncoda Technologies

Use of small molecules to rescue folding defective proteins
Patents for licensing Unismart - University of Padua Foundation

Rapid Computational Screening of Natural Products for COVID-19 Anti-Viral Agents
Innovative Products and Technologies Covid-19 Innovation Challenges by Innoget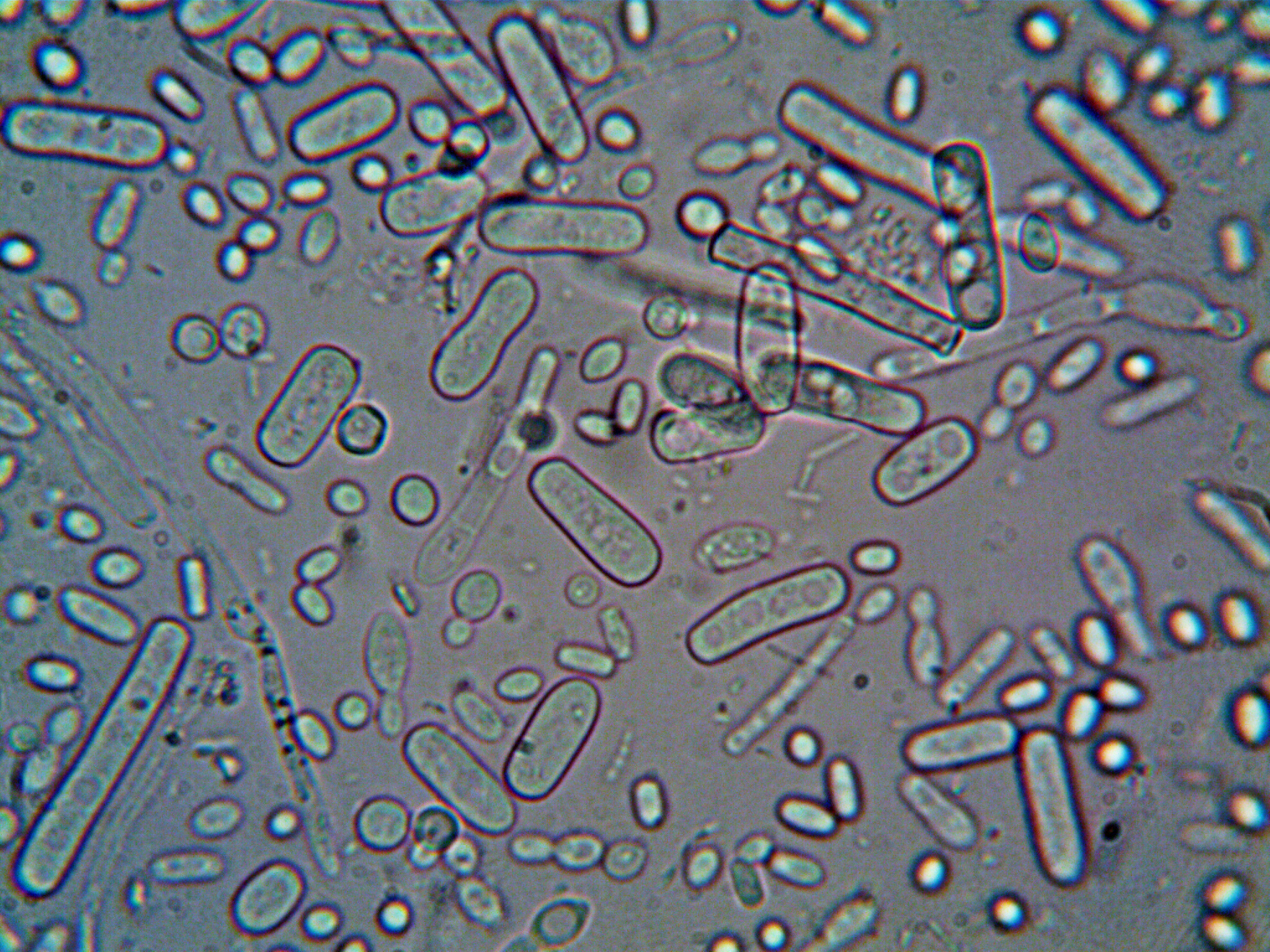

The use of microorganisms in the plant’s production
Patents for licensing Jagiellonian University

Strain textile sensor
Patents for licensing Universitat Politècnica de Catalunya - UPC

