Showing 1 to 15 of 2075 results

Multimodel Transcranial Optical Vascular Assessment
Patents for licensing Yeda![Commercially viable, environmentally sustainable conversion of, virtually any, organic carbon waste into high quality renewable en[…]](https://static4.innoget.com/uploads//93492a89c751ca7759b3b52e73d8328502a9e4cd.jpg)

Commercially viable, environmentally sustainable conversion of, virtually any, organic carbon waste into high quality renewable en[…]
Investment Opportunities in Startups and Spinoffs Reform Earth Technologies, LLC (RET)

Know-how in human language processing. Machine translation between European languages
Patents for licensing Universidad de Alicante

BLOOLA - Centralised communication, collaboration and task management
Innovative Products and Technologies EIT Digital

Nitric Oxide Chewing Gum Delivering in the Oral Cavity
Innovative Products and Technologies Per Os Biosciences

Prosody Based Speech Analysis
Patents for licensing Yeda

Method for manufacturing braided fiber reinforced composite dental posts
Patents for licensing Universidad de Granada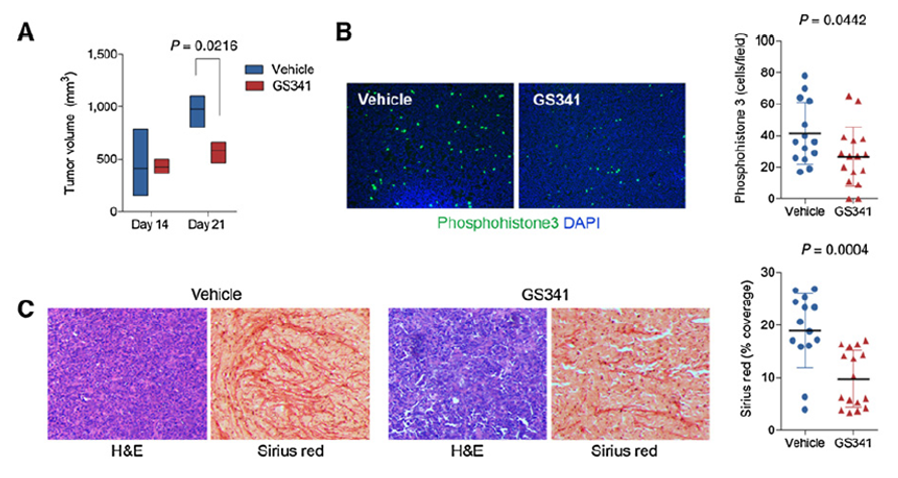

Treating Cancer and Collagen-Associated Diseases by Using Anti-LOXL-2 Antibody
Patents for licensing Yeda

MICROTRONICS - Enables your successful IoT business
Innovative Products and Technologies EIT Digital

TOrque Vectoring Electric Differential – TOVED
Patents for licensing Hibridesign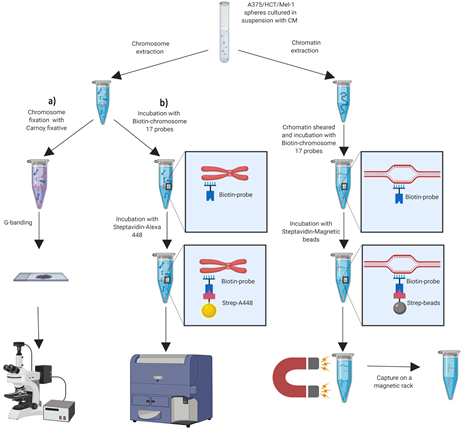

A simultaneous detection system of chromosomal alterations by sequencing, cytometry and imaging
Patents for licensing Universidad de Granada

JUSTSNAP - Turning Retail Receipts into Ecommerce Billboards
Innovative Products and Technologies EIT Digital

Procedure for the deoxygenation of sulfoxides quickly, efficiently and sustainably
Patents for licensing UNIVERSIDAD DE BURGOS

Multilingual Translator
Patents for licensing Universitat Politècnica de Catalunya - UPC