Never miss an update from Universidad de Alicante
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
The research group of Applied Electrochemistry and Electrocatalysis of the University of Alicante has developed an electric energy storage system. This system uses neutralization acid-base free energy for discharge process (use as battery), while charge process is performed by inverting previous reactions. The electrodic reactions are hydrogen evolution and oxidation reactions so no net hydrogen consumption exists.
The research group is looking for companies interested in licensing the technology, developing R & D projects to optimize this initial idea and/or to adapt this development to their needs for a future commercial exploitation of the patent.
New and innovative aspects
Main advantages of its use
Specifications
The current electric energy storage systems can be classified according the type of energy used. Thus, it can be considered systems using potential energy from compressed air (CAES, Compressed Air Energy Storage) or from pump-hydro (PHS, Pump-Hydro Storage), also using kinetic energy from flywheel (FWES, FlyWheel Energy Storage) or, finally, the most utilized ones where electrical energy is used.
These latter ones can be classified in two main types: supercapacitor systems and systems where electrical energy is stored in two reversible redox pairs. The charge capacity of these systems is defined by the mass of these reagents. This system can be represented by rechargeable batteries where reagents are static such as traditional lead-acid (P/A); nickel-cadmium system (Ni-Cd) and nickel metal hydride (Ni-MH), lithium-ion (Li-ion) or, redox flow batteries (RFB), where solutions associated to redox pairs are pumped to negative and positive electrode as appropriate. For this reason, these solutions are defined as posilyte and negalyte. In the same way, a type of redox battery is defined where water electrolysis producing hydrogen and oxygen using a renewable energy source, and produced hydrogen and oxygen is used in a fuel cell to obtain electric energy. The system, which is described in our patent, only uses hydrogen to perform the charge and discharge processes. Posilyte and
negalyte is acified and basified in charge process respectively. In discharge process, neutralization energy of these solutions is used. It is important to note that, hydrogen is consumed and produced in charge and discharge processes. Thus, theoretical net balance is close to zero and therefore, external supply of hydrogen is very low or negligible.
TECHNICAL DESCRIPTION
The developed technology consists of an electric energy storage system using neutralization energy of two solutions, one acidic and the other alkaline, separated by an ionic interchange membrane and hydrogen production/ self-supply is our chain gear in the present system.
Next, a diagram of the configuration of the electric energy storage system is shown:
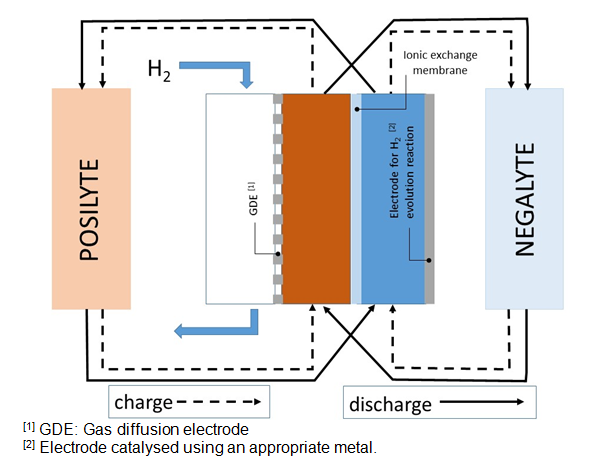
The operation of this system is based in the potential difference appearing when two electrodes are submerged in two solutions with different pHs, one highly acidic and the other one highly alkaline. Both solutions have a support electrolyte in concentrated enough for ionic transport through membrane,
performed by support electrolyte ions. In this sense, hydrogen production / self-supply is the chain gear of the present system.
Hydrogen oxidation reaction to hydronium ions and hydronium ion or water reduction reaction to hydrogen is produced, both processes are very reversible. The acidity and basicity of starting solutions can be neutralized obtaining electric energy (discharge) or can be increased providing electric energy (charge).
The reactions which are involved in charge and discharge processes are the following:
Discharge process:
H+ y OH- is consumed (in different electrodes, certainly) and H2O is globally produced.
Charge process:
Posilyte and negalyte are acidified and basified.
Applications
Intellectual property status
This technology is protected by patent application.
Current development status
A proof of concept has been tested in a laboratory scale.
Desired business relationship
Companies interested in acquisition of this technology are looked for. In this case, a commercial prototype could be developed by:
Ahead of the current Coronavirus outbreak, Innoget is fully committed to contributing to mobilizing scientific and expert communities to find a real solution to the Covid-19 pandemic. Therefore, we're supporting worldwide calls and programs that could help in any aspects of the coronavirus crisis.
Is your organization promoting or looking for innovation or research initiatives to mitigate the Covid-19 outbreak? Email us at covid19@innoget.com to list them.
Channeled through Innoget's online open innovation network, initiatives in the health, virology, medicine, or novel technologies applied to human health, among others, are listed and disseminated to Innoget members -ranging from hospitals, research institutes, scientists, businesses, and public administrations- and innovation partners worldwide.
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
Send a request for information
to Universidad de Alicante
Technology Offers on Innoget are directly posted
and managed by its members as well as evaluation of requests for information. Innoget is the trusted open innovation and science network aimed at directly connect industry needs with professionals online.
Need help requesting additional information or have questions regarding this Technology Offer?
Contact Innoget support