Showing 1 to 15 of 2075 results


Enzymatic production of 2,5-furandicarboxylic acid from 5-methoxymethylfurfura
Patents for licensing Consejo Superior de Investigaciones Científicas

TU Dublin have developed AI assisted hardware/software package
Innovative Products and Technologies TU Dublin Hothouse

Use of BCL7A as molecular target for DLBCL drug screening & development.
Patents for licensing Universidad de Granada

Nanoparticles of functionalized silica for the removal of arsenate
Patents for licensing Universidad Andres Bello

Thin Film Flexible and Stretchable Pressure Sensing Mat
Innovative Products and Technologies IPI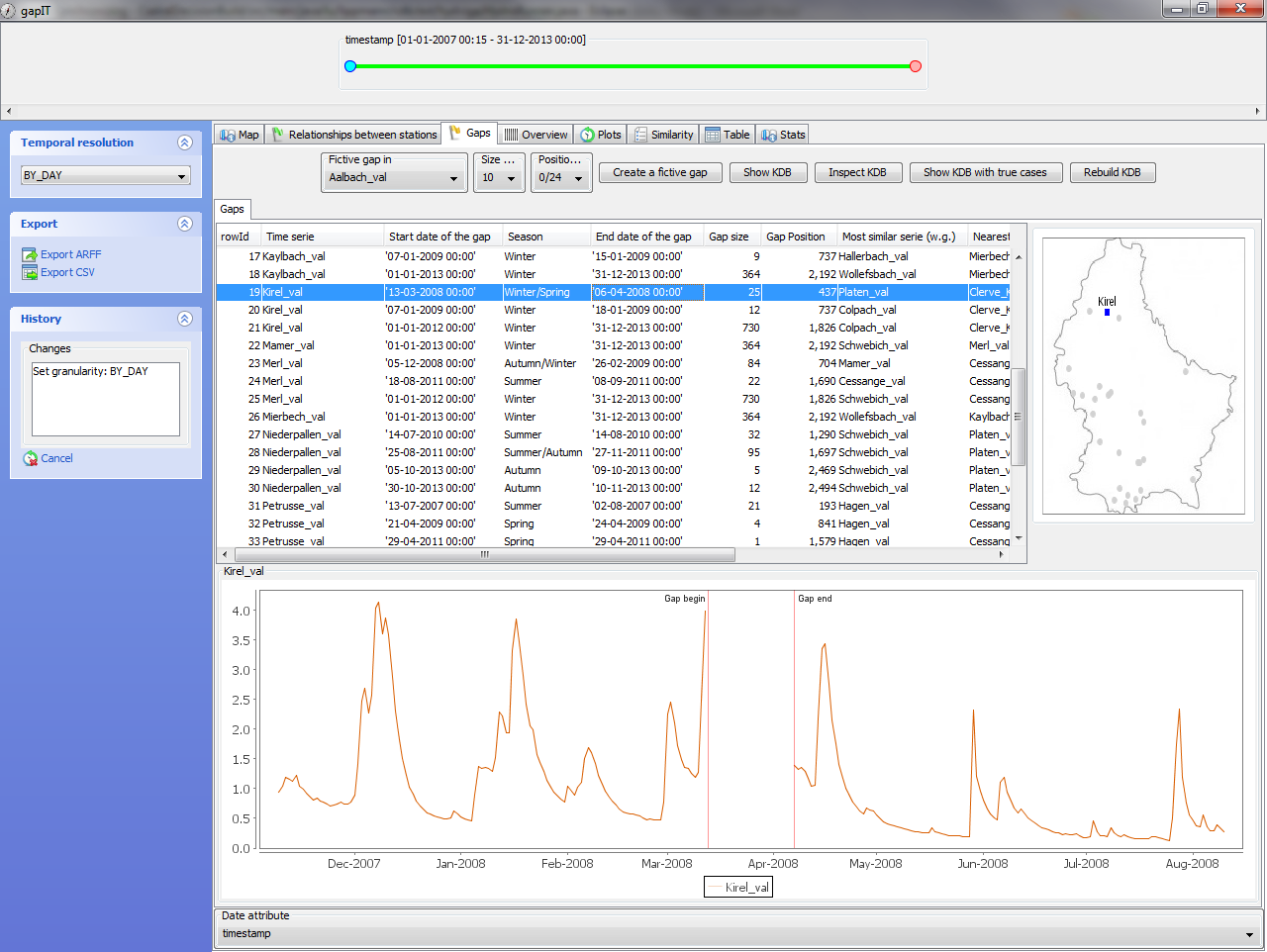

An interactive software tool to deal with missing values (gap filling) in hydrological time series for water management
Innovative Products and Technologies Luxembourg Institute of Science and Technology (LIST)

‘eEarlyCare’ therapeutic intervention program
Patents for licensing UNIVERSIDAD DE BURGOS

Multilingual Translator
Patents for licensing Universitat Politècnica de Catalunya - UPC

Synthetic data for Computer Vision applications
Innovative Products and Technologies LexSet.ai

Ultra low power MODBUS Temperature, Humidity, Pressure sensor
Innovative Products and Technologies Barani Design

Self-compacting concrete with recycled concrete aggregate and low shrinkage
Patents for licensing UNIVERSIDAD DE BURGOS

A system for Red Seaweed culture
Patents for licensing Universidad Andres Bello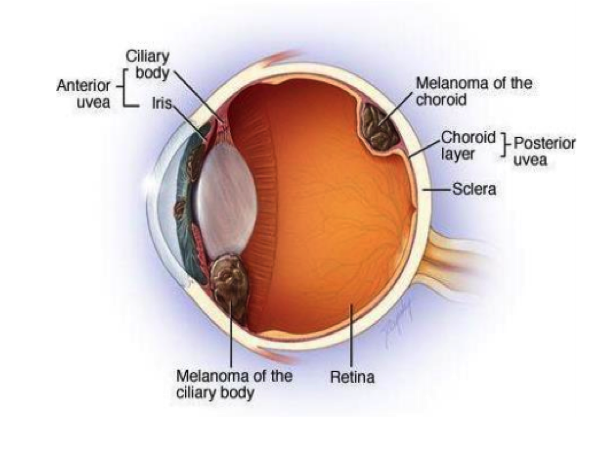

Companion diagnostic for cutaneous and uveal melanoma
Innovative Products and Technologies IDIBELL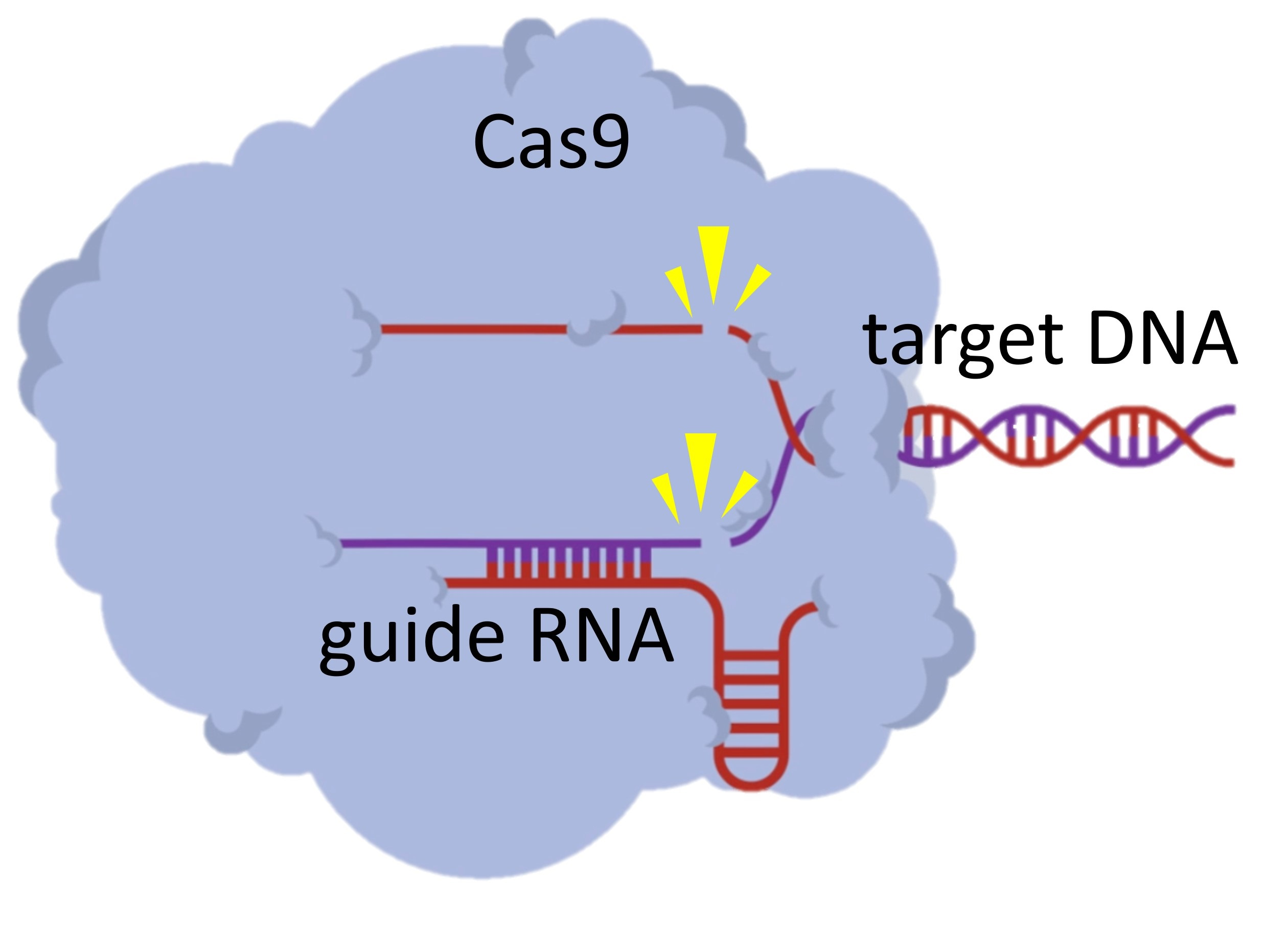

Method for an accurate simultaneous detection of multiple viruses in samples
Patents for licensing CSIC - Consejo Superior de Investigaciones Científicas

